Abstract
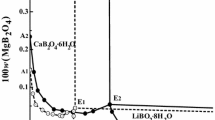
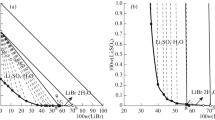
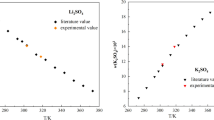
Experimental studies on the solubilities and physicochemical properties including density, refractive index and pH value in the ternary systems (LiBO2 + Li2CO3 + H2O) at 288.15 and 298.15 K were determined with the method of isothermal dissolution equilibrium. Based on the experimental results, the phase diagrams and their corresponding physicochemical properties versus composition diagram in the system were plotted. In the phase diagrams of the ternary system at 288.15 and 298.15 K, there are one eutectic point and two crystallization regions corresponding to lithium metaborate octahydrate (LiBO2 · 8H2O) and lithium carbonate (Li2CO3), respectively. This system at both temperatures belongs to hydrate type I, and neither double salt nor solid solution was found. A comparison of the phase diagrams for this ternary system at 288.15 and 298.15 K shows that the solid phase numbers and exist minerals are the same, and the area of crystallization region of Li2CO3 is increased obviously with the increasing temperature while that of LiBO2 · 8H2O is decreased. The physicochemical properties (density, pH value and refractive index) of the solutions of the ternary system at two temperatures changes regularly with the increasing lithium carbonate concentration. The calculated values of density and refractive index using empirical equations of the ternary system are in good agreement with the experimental values.
Similar content being viewed by others
References
X. Y. Zheng, Y. Tang, and Y. Xu, Tibet Saline Lake (Chin. Sci. Technol., Beijing, 1988).
S. Q. Wang and T. L. Deng, J. Chem. Thermodyn. 40, 1007 (2008).
T. L. Deng, H. J. Yin, and Y. F. Guo, J. Chem. Eng. Data 56, 3585 (2011).
T. L. Deng, H. J. Yin, and D. C. Li, J. Chem. Eng. Data 54, 498 (2008).
Y. F. Guo, D. L. Gao, H. J. Han, et al., Fluid Phase Equilib. 358, 56 (2013).
Y. F. Guo, Y. H. Liu, Q. Wang, et al., J. Chem. Eng. Data 58, 2763 (2013).
D. L. Gao, Q. Wang, Y. F. Guo, et al., Fluid Phase Equilib. 371, 121 (2014).
S. Q. Wang and T. L. Deng, J. Chem. Eng. Data 55, 4211 (2010).
J. Gao and T. L. Deng, J. Chem. Eng. Data 56, 1452 (2011).
S. Q. Wang, F. Y. Guo, D. C. Li, et al., Thermochim. Acta 601, 75 (2015).
J. E. Teeple, The Industrial Development of Seales Lake Brines: With Equilibrium Data (The Chemical Catalog Company, New York, 1929).
Analytical laboratory of Qinghai institute of salt lakes at CAS, The Analyses of Brines and Salts, 2nd ed. (Chin. Science, Beijing, 1988).
C. H. Fang, J. Salt Lake Res. 2, 15 (1990).
J. M. Speight, Lange’s Handbook of Chemistry, 16th ed. (McGraw-Hill, New York, 2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Wang, Sq., Guo, Yf., Yang, Js. et al. Solid-liquid phase equilibria in the ternary system (LiBO2 + Li2CO3 + H2O) at 288.15 and 298.15 K. Russ. J. Phys. Chem. 89, 2190–2196 (2015). https://doi.org/10.1134/S0036024415120286
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024415120286