Abstract
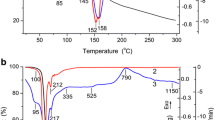
Chemical reactions and physical transformations that occur upon heating aluminum hydride (AlH3, alane), stored for 25 years, in the temperature range of 50–1200°C in an atmosphere of nitrogen, argon, and air are studied by means of thermogravimetric analysis and differential scanning calorimetry. The heat of thermal decomposition and the hydrogen content are determined for the AlH3 samples and are found to be 318 ± 25 J/g and 9.32 ± 0.24 wt %, respectively. It is established that the estimated enthalpy of formation of AlH3 in stoichiometric composition (Δf H ≈ −10.3 kJ/mol) agrees with the literature data. After the release of hydrogen, the mass of the precipitate increases by 0.5 ± 0.3%, relative to the initial mass of the AlH3 samples; the most likely reason for this effect is the adsorption of nitrogen (argon) in the micropores and mesopores that form. Thermal phenomena associated with the crystallization of the amorphous aluminum that forms after hydrogen is released from the alane particles are analyzed. It is established that the aluminum contained in initial AlH3 samples is almost completely transformed into aluminum nitride and oxide (AlN and Al3O3) upon heating to 1200°C in nitrogen and air, respectively.
Similar content being viewed by others
References
J. Graetz, J. J. Reilly, V. A. Yartys, et al., J. Alloys Compd. 509, S517 (2011).
J. Graetz, J. J. Reilly, J. G. Kulleck, and R. C. Bowman, J. Alloys Compd. 446–447, 271 (2007).
J. Graetz, J. J. Reilly, G. Sandrock, et al., Formal Report BNL-7733-2006 (Brookhaven National Laboratory, 2006).
I. M. K. Ismail and T. W. Hawkins, Technical Paper of Air Force Research Laboratory (Edwards, CA, USA, 2005. http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA440306
J. P. Maehlen, V. A. Yartis, R. V. Denis, et al., J. Alloys Compd. 446–447, 280 (2007).
J. Graetz and J. J. Reilly, J. Alloys Compd. 424, 262 (2006).
V. A. Kireev, Methods of Applied Calculations in Thermodynamics of Chemical Reactions (Khimiya, Moscow, 1975) [in Russian].
Yu. K. Tovbin, Nanotechnol. Russ. 5, 715 (2010).
A. P. Karnaukhov, Adsorption. Texture of Disperse and Porous Materials (Nauka, Novosibirsk, 1999) [in Russian].
M. S. Dulya, V. N. Fokin, and B. P. Tarasov, Al’tern. Energet. Ekol., No. 9 (53), 25 (2007).
A. V. Korshunov, Izv. Tomsk. Politekh. Univ. 316, 17 (2010).
A. P. Il’in, A. A. Gromov, and L. O. Tolbanova, Fundam. Issled., No. 4, 13 (2008).
L. A. Akashev, N. A. Popov, V. A. Kochedykov, and V. G. Shevchenko, Tech. Phys. Lett. 39, 154 (2013).
C.-N. Lin and S.-L. Chung, J. Mater. Res. 16, 2200 (2001).
M. R. Ranade, F. Tessier, A. Navrotsky, and R. Marchand, J. Mater. Res. 16, 2824 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.M. Milekhin, A.A. Koptelov, A.A. Matveev, Yu.N. Baranets, D.A. Bakulin, 2015, published in Zhurnal Fizicheskoi Khimii, 2015, Vol. 89, No. 7, pp. 1068–1073.
Rights and permissions
About this article
Cite this article
Milekhin, Y.M., Koptelov, A.A., Matveev, A.A. et al. Studying aluminum hydride by means of thermal analysis. Russ. J. Phys. Chem. 89, 1141–1145 (2015). https://doi.org/10.1134/S0036024415070250
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024415070250