Abstract
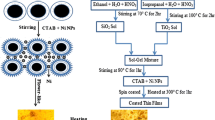
Manganese-doped nanostructured composites based on TiO2 were obtained by the template sol–gel method. By a set of investigation techniques, it was established that manganese doping leads to the appearance of a rutile phase, which grows with increasing manganese content. It was found that during the template sol–gel synthesis of the TiO2/Mn composite, the doping element is not only incorporated into the titanium dioxide structure, but also forms separate phases on its surface. Study of the magnetic characteristics of the obtained composites revealed ferromagnetic ordering at room temperature, while lowering the temperature leads to an increase in the coercive force and appearance of the ferromagnet/antiferromagnet type exchange interaction. A magnetic transition at about 43 K, associated with the transfer of Mn3O4 nanoparticles to the ferromagnetic state, was detected in the composites. Undoped titanium dioxide showed a high photocatalytic activity in the visible spectral range, providing 98% degree of indigo carmine degradation. Manganese doping of TiO2 microtubes inhibited the photocatalytic activity.








Similar content being viewed by others
REFERENCES
M. A. Uimin, A. S. Minin, A. E. Ermakov, et al., Mezhd. Zh. Prikl. Fund. Issled. 12, 49 (2017). https://applied-research.ru/ru/article/view?id=11961
S. Sharma, S. Chaudhary, S. C. Kashyap, and S. K. Sharma, J. Appl. Phys. 109, 083905 (2011). https://doi.org/10.1063/1.3567938
Ding Peng, Liu Fa-Min, Zhou Chang-Cang, et al., Chin. Phys. B 19, 1181102 (2010). https://doi.org/10.1088/1674-1056/19/11/118102
R. I. Khaibullin, L. R. Tagirov, Sh. Z. Ibragimov, et al., Uch. Zap. Kazan. Gos. Univ. 149, 31 (2007).
M. A. Uymin, A. S. Minin, A. Y. Yermakov, et al., Lett Mater. 9, 91 (2019). https://doi.org/10.22226/2410-3535-2019-1-91-96
O. A. Shilova, A. S. Kovalenko, A. M. Nikolaev, et al., Russ. J. Inorg. Chem. 66, 665 (2021). https://doi.org/10.1134/S0036023621050181
T. Ochiai and A. Fujishima, J. Photochem. Photobiol. C 13, 247 (2012). https://doi.org/10.1016/j.jphotochemrev.2012.07.001
A. Fujishima and K. Honda, Nature 238, 37 (1972). https://doi.org/10.1038/238037a0
J. Wang, Y. Guo, B. Liu, et al., Ultrason. Sonochem. 18, 177 (2011). https://doi.org/10.1016/j.ultsonch.2010.05.002
H. A. Reddam, R. Elmail, S. C. Lloria, et al., Ceram. Vidrio 59, 138 (2019). https://doi.org/10.1016/j.bsecv.2019.09.005
L. Sangaletti, M. C. Mozzati, G. Drera, et al., Phys. Rev. B: Condens. Matter. 78, 075210 (2008). https://doi.org/10.1103/PhysRevB.78.075210
N. Akdogan, A. Nefedov, H. Zabel, et al., J. Phys. D: Appl. Phys. 42, 115005 (2009). https://doi.org/10.1088/0022-3727/42/11/115005
W. B. Mi, E. Y. Jiang, and H. L. Bai, J. Magn. Magn. Mater. 321, 2472 (2009). https://doi.org/10.1016/j.jmmm.2009.03.017
S. Bhattacharyya, A. Pucci, D. Zitoun, et al., Nanotecnology 19, 495711 (2008). https://doi.org/10.1088/0957-4484/19/49/495711
Z. M. Tian, S. I. Yan, Y. Q. Wang, et al., J. Phys. D: Appl. Phys. 41, 055006 (2008). https://doi.org/10.1088/0022-3727/41/5/055006
N. H. Hong, J. Sakaib, W. Prellierc, et al., Physica B: Condens. Matter. 355, 295 (2005). https://doi.org/10.1016/j.physb.2004.11.021
J. Osterwalder, T. Droubaya, T. Kaspara, et al., Thin Solid Films 484, 289 (2005). https://doi.org/10.1016/j.tsf.2005.02.028
V. V. Zheleznov, Y. V. Sushkov, E. I. Voit, et al., J. Appl. Spectrosc. 81, 983 (2015). https://doi.org/10.1007/s10812-015-0039-6
D. P. Opra, S. V. Gnedenkov, S. L. Sinebryukhov, et al., Elektrokhim. Energ. 19, 123 (2019). https://doi.org/10.18500/1608-4039-2019-19-3-123-140
S. A. Kukharenko, A. E. Shilo, P. P. Itsenko, et al., J. Superhard Mater. 32, 396 (2010). https://doi.org/10.3103/S1063457610060055
J. Sackey, M. Akbari, R. Morad, et al., J. Alloys Compd. 854, 156987 (2021). https://doi.org/10.1016/j.jallcom.2020.156987
J. M. D. Coey, M. Venkatesan, and C. B. Fitzgerald, Nat. Mater. 4, 173 (2005). https://doi.org/10.1038/nmat1310
A. E. Ermakov, M. A. Uimin, A. V. Korolev, et al., Phys. Solid State 59, 469 (2017). https://doi.org/10.1134/S1063783417030106
K. Oganisian, A. Hreniak, A. Sikora, et al., Proc. Appl. Ceram. 9, 43 (2015). https://doi.org/10.2298/PAC1501043O
M. Wang, B. Nie, K.-K. Yee, et al., Chem. Commun. 52, 2988 (2016). https://doi.org/10.1039/C5CC09176D
A. Šuligoj, I. Arčon, M. Mazaj, et al., J. Mater. Chem. A 6, 9882 (2018). https://doi.org/10.1039/c7ta07176k
M. Lešnik, D. Verhovšek, N. Veronovski, et al., Mater. Technol. 52, 411 (2018). https://doi.org/10.17222/mit.2017.012
S. M. Chang and W. S. Liu, Appl. Catal. B 156–157, 466 (2014). https://doi.org/10.1016/j.apcatb.2014.03.044
H. Sudrajat, S. Babel, A. T. Ta, et al., J. Phys. Chem. Solids 144, 109517 (2020). https://doi.org/10.1016/j.jpcs.2020.109517
J. Lee, A. Gopalan, G. Saianand, et al., Nanomaterials 10, 456 (2020). https://doi.org/10.3390/nano10030456
X. Ma, W. Zhou, and Y. Chen, Materials 10, 631 (2017). https://doi.org/10.3390/ma10060631
Z. Xu, C. Li, N. Fu, et al., J. Appl. Electrochem. 48, 1197 (2018). https://doi.org/10.1007/s10800-018-1198-y
R. Chauhan, A. Kumar, and R. P. Chaudhary, Spectrochim. Acta A 98, 256 (2019). https://doi.org/10.1016/j.saa.2012.08.009
L. N. Obolenskaya, E. N. Domoroshchina, E. V. Savinkina, et al., Fund. Issled. 1–3, 796 (2013).
ACKNOWLEDGMENTS
The study was performed using equipment of the center for collective use “Far Eastern Center for Structural Research” of the Institute of Chemistry, Far Eastern Branch, Russian Academy of Sciences.
Funding
This study was performed within the framework of the State assignment of the Institute of Chemistry, Far Eastern Branch, Russian Academy of Sciences, topic no. 0205-2021-0002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Tkachenko, I.A., Marchenko, Y.V., Vasilyeva, M.S. et al. Manganese-Doped Composites Based on Titanium Dioxide: Template Sol–Gel Synthesis, Structure, and Properties. Russ. J. Inorg. Chem. 67, 1339–1347 (2022). https://doi.org/10.1134/S0036023622090169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622090169