Abstract
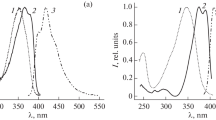
A simple method is proposed for the preparative synthesis of substituted benzoylacetonates of boron difluoride in one step from donor-substituted arenes by acetoacetylation under the action of boron trifluoride. The effect of the nature of alkyl and aryl substituents on the phenyl ring of boron difluoride benzoylacetonates on their spectral and luminescent properties is analyzed. It is shown that alkyl substituents insignificantly increase the luminescence quantum yield. A significant increase in the luminescence quantum yield up to 0.75 and a significant bathochromic shift up to 536 nm are achieved with an increase in the π-system of the molecule: the transition from phenyl to naphthyl, diphenyl, and anthracyl.





Similar content being viewed by others
REFERENCES
N. A. Bumagina, A. Y. Kritskaya, E. V. Antina, et al., Russ. J. Inorg. Chem. 63, 1326 (2018). https://doi.org/10.1134/S0036023618100030
E. V. Antina, M. B. Berezin, A. I. V’yugin, et al., Russ. J. Inorg. Chem. 67, 321 (2022). https://doi.org/10.1134/S0036023622030032
F. Wang, D. L. Song, and D. A. Dickie, J. Fluoresc. 31, 39 (2021). https://doi.org/10.1007/s10895-020-02626-8
S. Lee, S. Kwak, K.-K. Wan, et al., ECS J. Solid State Sci. Technol. 110, 086007 (2020). https://doi.org/10.1149/2162-8777/ac1d60
Q.-Z. H. Zhang, P.-Z. Chen, L.-Y. Niu, et al., Mater. Chem. Front. 4, 285 (2020). https://doi.org/10.1039/C9QM00672A
A. Loudet and K. Burgess, Chem. Rev. 107, 4891 (2007). https://doi.org/10.1021/cr078381n
M. Collot, Mater. Horiz. 8, 50 (2021). https://doi.org/10.1039/d0mh01186j
A. Kamkaew, S. H. Lim, H. B. Lee, et al., Chem. Soc. Rev. 42, 77 (2013). https://doi.org/10.1039/C2CS35216H
C. I. Nieto, M. P. Cabildo, M. P. Cornago, et al., Molecules 20, 15643 (2015). https://doi.org/10.3390/molecules200915643
I. V. Svistunova, N. P. Shapkin, and O. V. Nikolaeva, Russ. J. Gen. Chem. 72, 899 (2002). https://doi.org/10.1023/A:1020426105849
M. Halik and H. Hartmann, Chem.-Eur. J. 5, 2511 (1999).
V. F. Traven, T. A. Chibisova, and A. V. Manaev, Dyes Pigments 58, 41 (2003). https://doi.org/10.1016/S0143-7208(03)00022-6
A. Goel, A. B. Kunnumakkara, and B. B. Aggarwal, Biochem. Pharmacol. 75, 787 (2008). https://doi.org/10.1016/j.bcp.2007.08.016
M. Mayadevi, D. R. Sherin, and V. S. Keerthi, et al., Bioorg. Med. Chem. 20, 6040 (2012). https://doi.org/10.1016/j.bmc.2012.08.029
H. Hatchera, R. Planalp, J. Chob, et al., Cell. Mol. Life Sci. 65, 1631 (2008). https://doi.org/10.1007/s00018-008-7452-4
I. Benoit, M. Asther, G. Sulzenbacher, et al., FEBS Lett. 580, 5815 (2006). https://doi.org/10.1016/j.febslet.2006.09.039
J. A. Hermoso, J. Sanz-Aparicio, R. Molina, et al., J. Mol. Biol. 338, 495 (2004). https://doi.org/10.1016/j.jmb.2004.03.003
K. Liu, J. Chen, J. Chojnacki, et al., Tetrahedron Lett. 54, 2070 (2013). https://doi.org/10.1016/j.tetlet.2013.02.015
N. M. D. Brown and P. Bladon, J. Chem. Soc. A 526 (1969). https://doi.org/10.1039/J19690000526
H. Meerwein and D. Vossen, J. Prakt. Chem. 141, 149 (1934). https://doi.org/10.1002/prac.19341410503
C. R. Hauser, F. W. Swamer, and J. T. Adams, The Acylation of Ketones to Form β-Diketones or β-Keto Aldehydes. Organic Reactions (2011). https://doi.org/10.1002/0471264180.or008.03
C. R. Hauser, F. C. Frostik, and E. H. Man, J. Am. Chem. Soc. 74, 3231 (1952). https://doi.org/10.1021/ja01133a0082
G. Gorlitz, H. Hartmann, J. Kossanyi, et al., Ber. Bunsen-Ges. Phys. Chem. 102, 1449 (1998). https://doi.org/10.1002/(SICI)1521-3897(199902)34-1:23.0.CO;2-A
G. Gorlitz, H. Hartmann, and B. Nuber, J. Pract. Chem. 341, 167 (1999). https://doi.org/10.1002/(SICI)1521-3897(199902)341:2-3.0.CO;2-A
Y. Dromzee, J. Kossanyi, V. Wintgens, et al., Z. Kristallogr. 212, 372 (1997). https://doi.org/10.1524/zkri.1997.212.5.372
J. N. Demas and G. A. Crosby, J. Phys. Chem. J. Phys. Chem. 75, 991 (1971). https://doi.org/10.1021/j100678a001
A. Weissberger, E. S. Proskauer, J. A. Riddick, and E. E. Toops, Organic Solvents. Physical Properties and Methods of Purifiation (Interscience Publ., New York, 1955).
V. E. Karasev and O. A. Korotkich, Russ. J. Inorg. Chem. 31, 869 (1986).
E. V. Fedorenko, A. G. Mirochnik, A. V. Gerasimenko, et al., J. Photochem. Photobiol., A 412, 113220 (2021). https://doi.org/10.1016/j.jphotochem.2021.113220
B. V. Bukvetskii, E. V. Fedorenko, and A. G. Mirochnik, J. Struct. Chem. 52, 221 (2011). https://doi.org/10.1134/s0022476611010331
E. V. Fedorenko, B. V. Bukvetskii, A. G. Mirochnik, et al., J. Lumin. 130, 756 (2010). https://doi.org/10.1016/j.jlumin.2009.11.027
B. V. Bukvetskii, E. V. Fedorenko, and A. G. Mirochnik, J. Struct. Chem. 51, 785 (2010). https://doi.org/10.1007/s10947-010-0118-8
B. V. Bukvetskii, E. V. Fedorenko, and A. G. Mirochnik, Struct. Chem. 51, 545 (2010). https://doi.org/10.1007/s10947-010-0079-y
March’s Advanced Organic Chemistry—Reactions, Mechanisms, and Structure, Ed. by M. B. Smith and J. March (Wiley Interscience, 2007).
N. Donaldson, The Chemistry and Technology of Naphthalene Compounds, Ed. by E. Arnold (London, 1958).
E. V. Fedorenko, A. G. Mirochnik, and A. Yu. Beloliptsev, J. Lumin. 196, 316 (2018). https://doi.org/10.1016/j.jlumin.2017.12.071
Funding
The work was supported by the Ministry of Science and Higher Education of the Russian Federation, State Assignment no. 0205-2021-0001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Mirochnik, A.G., Puzyrkov, Z.N., Fedorenko, E.V. et al. Synthesis and Spectroscopy of Substituted Benzoylacetonates of Boron Difluoride. Russ. J. Inorg. Chem. 67, 1425–1432 (2022). https://doi.org/10.1134/S003602362209008X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602362209008X