Abstract
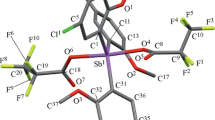
In a crystal of tris(2-methoxy-5-chlorophenyl)antimony benzene solvate (5-Cl-2-MeO-C6H3)3Sb (I) ⋅ 1/2С6Н6 synthesized from SbCl3 and 2-methoxy-5-chlorophenyllithium with recrystallization from benzene, the antimony atoms are in a trigonal environment of ligands with the oxygen atoms of MeO groups coordinated to the metal atom (intramolecular Sb∙∙∙OМе distances are 3.053(1), 3.054(1), and 3.011(1) Å, CN = 3 + 3). The effect of tert-butyl hydroperoxide on a mixture of tris(2-methoxy-5-chlorophenyl)antimony and bromoacetic, 3-fluorophenylacetic, and 2,3-difluorobenzoic acids in ether leads to the formation of trigonal bipyramidal triarylantimony dicarboxylates (5-Cl-2-MeO-C6H3)3Sb[OC(O)R]2, where R = CH2Br (II), CH2C6H4F-3 (III), C6H3F2-2,3 (IV) with carboxylate ligands in apical positions. In crystals of compounds II–IV, the metal atom is additionally coordinated to the oxygen atoms of О=С and МеО groups.




Similar content being viewed by others
REFERENCES
Cambridge Crystallographic Data Center (2019). http://www.ccdc.cam.ac.uk.
K. Onishi, M. Douke, T. Nakamura, et al., J. Inorg. Biochem. 117, 77 (2012). https://doi.org/10.1016/j.jinorgbio.2012.09.009
D. Copolovici, F. Isaia, H. J. Breunig, et al., RSC Advances 4, 26569 (2014). https://doi.org/10.1039/C4RA03482A
D. Copolovici, V. R. Bojan, C. I. Rat, et al., Dalton Trans. 39, 6410 (2010). https://doi.org/10.1039/C003318A
S. Okajima, S. Yasuike, N. Kakusawa, et al., J. Organomet. Chem. 656, 234 (2002). https://doi.org/10.1016/S0022-328X(02)01622-4
H. Yamamichi, S. Matsukawa, S. Kojima, et al., Heteroat. Chem. 22, 553 (2011). https://doi.org/10.1002/hc.20721
T. Reznicek, L. Dostal, A. Ruzicka, et al., Appl. Organomet. Chem. 26, 237 (2012). https://doi.org/10.1002/aoc.2845
T. Obata, M. Matsumura, M. Kawahata, et al., J. Organomet. Chem. 807, 17 (2016). https://doi.org/10.1016/j.jorganchem.2016.02.008
Y. Matano, H. Nomura, T. Hisanaga, et al., Organometallics 23, 5471 (2004). https://doi.org/10.1021/om0494115
V. V. Sharutin and O. K. Sharutina, Russ. J. Inorg. Chem. 60, 1491 (2015). https://doi.org/10.1134/S0036023615120219
V. V. Sharutin, V. S. Senchurin, O. K. Sharutina, et al., Russ. Coord. Chem. 37, 781 (2011). https://doi.org/10.1134/S1070328411090089
V. V. Sharutin, V. S. Senchurin, O. K. Sharutina, et al., Russ. J. Gen. Chem. 81, 2102 (2011). https://doi.org/10.1134/S1070363211100100
V. V. Sharutin, V. S. Senchurin, O. K. Sharutina, et al., Russ. J. Inorg. Chem. 56, 1561 (2011). https://doi.org/10.1134/S0036023611100196
Y. Matano, H. Nomura, and H. Suzuki, Inorg. Chem. 39, 1340 (2000). https://doi.org/10.1021/ic991120e
Y. Matano, H. Nomura, and H. Suzuki, Inorg. Chem. 41, 1940 (2002). https://doi.org/10.1021/ic0110575
V. V. Sharutin, O. K. Sharutina, V. S. Senchurin, et al., Russ. J. Gen. Chem. 82, 1665 (2012). https://doi.org/10.1134/S1070363212100064
V. V. Sharutin, O. K. Sharutina, and V. S. Senchurin, Russ. J. Inorg. Chem. 59, 326 (2014). https://doi.org/10.1134/S0036023614040202
T. Iftikhar, M. K. Rauf, S. Sarwar, et al., J. Organomet. Chem. 851, 89 (2017). https://doi.org/10.1016/j.jorganchem.2017.09.002
R. Mushtaq, M. K. Rauf, M. Bond, et al., Appl. Organomet. Chem. 30, 465 (2016). https://doi.org/10.1002/aoc.3456
R. Mushtaq, M. K. Rauf, M. Bolte, et al., Appl. Organomet. Chem. 31, e3606 (2017). https://doi.org/10.1002/aoc.3606
M. I. Ali, M. K. Rauf, A. Badshah, et al., J. Chem. Soc., Dalton Trans. 42, 16733 (2013). https://doi.org/10.1039/c3dt51382c
Y. Q. Ma, L. Yu, and J. S. Li, Heteroat. Chem. 13, 299 (2002). https://doi.org/10.1002/hc.10033
A. Islam, SilvaJ. G. Da, F. M. Berbet, et al., Molecules 19, 6009 (2014). https://doi.org/10.3390/molecules19056009
R. C. Liu, Y. Q. Ma, L. Yu, et al., Appl. Organomet. Chem. 17, 662 (2003). https://doi.org/10.1002/aoc.491
J. S. Li, R. C. Liu, X. B. Chi, et al., Inorg. Chim. Acta 357, 2176 (2004). https://doi.org/10.1016/j.ica.2003.12.012
Y. Ma, J. Li, Z. Xuan, et al., J. Organomet. Chem. 620, 235 (2001). https://doi.org/10.1016/S0022-328X(00)00799-3
J. S. Li, Y. Q. Ma, J. R. Cui, et al., Appl. Organomet. Chem. 15, 639 (2001). https://doi.org/10.1002/aoc.200
X.-Y. Zhang, L. Cui, X. Zhang, et al., J. Mol. Struct. 1134, 742 (2017). https://doi.org/10.1016/j.molstruc.2017.01.039
K. Lowe and R. Powell, J. Fluorine Chem. 109, 1 (2001). https://doi.org/10.1016/S0022-1139(01)00371-2
B. E. Smart, J. Fluorine Chem. 109, 3 (2001). https://doi.org/10.1016/S0022-1139(01)00375-X
O. K. Sharutin and A. N. Sharutina, Efremov, and P. A. Slepukhin, Russ. J. Inorg. Chem. 65, 992. https://doi.org/10.1134/S0036023620070190
B. K. Park and N. R. Kitteringham, Drug Metab. Rev. 26, 605 (1994). https://doi.org/10.3109/03602539408998319
P. Maienfisch and R. G. Hall, Chimia Int. J. Chem. 58, 93 (2004). https://doi.org/10.2533/000942904777678091
SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System (Bruker, 1998).
SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data (Bruker, 1998).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009). https://doi.org/10.1107/S0021889808042726
E. A. Adams, J. W. Kolis, and W. T. Pennington, Acta Crystallogr., Sect. C 46, 917 (1990). https://doi.org/10.1071/C96042
A. N. Sobolev, I. P. Romm, V. K. Belskii, et al., J. Organomet. Chem. 179, 153 (1979). https://doi.org/10.1016/S0022-328X(00)95217-3
B. Cordero, V. Gomez, A. E. Platero-Prats, et al., Dalton Trans. 21, 2832 (2008). https://doi.org/10.1039/B801115J
M. Mantina, A. C. Chamberlin, R. Valero, et al., J. Phys. Chem. A 113, 5806 (2009). https://doi.org/10.1021/jp8111556
V. V. Sharutin, O. K. Sharutina, V. S. Senchurin, et al., Russ. J. Gen. Chem. 79, 2131 (2009). https://doi.org/10.1134/S1070363209100107
E. V. Artem’eva, V. V. Sharutin, O. K. Sharutina, et al., Russ. J. Inorg. Chem. 65, 22 (2020). https://doi.org/10.1134/S0036023620010039
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Sharutin, V.V., Sharutina, O.K. Tris(2-metoxy-5-chlorophenyl)antimony Dicarboxylates (5-Cl-2-MeO-C6H3)3Sb[OC(O)R]2, Where R = CH2Br, CH2C6H4F-3, and C6H3F2-2,3: Synthesis and Structure. Russ. J. Inorg. Chem. 66, 361–366 (2021). https://doi.org/10.1134/S0036023621030153
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621030153