Abstract—
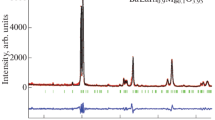
The BaLaIn0.9Zn0.1O3.95 phase with a Ruddlesden–Popper block layer structure (space group Pbca) was obtained for the first time and certified by X-ray diffraction. In a dry air atmosphere (pO2 = 0.21 atm, pH2O = 3.5 × 10−5 atm), the compound predominantly exhibited the oxygen ion type of conductivity. The acceptor doping of the BaLaInO4 cation sublattice with Zn2+ ions was found to induce an increase in the electrical conductivity due to the formation of oxygen vacancies in the structure. With increasing ionic radius of the dopant, the oxygen ion conductivity increased because of increasing free volume of the crystal lattice.




Similar content being viewed by others
REFERENCES
J. B. Goodenough, Annu. Rev. Mater. Res. 33, 91 (2003). https://doi.org/10.1146/annurev.matsci.33.022802.091651
V. V. Kharton, F. M. B. Marques, and A. Atkinson, Solid State Ionics 174, 135 (2004). https://doi.org/10.1016/j.ssi.2004.06.015
Perovskite Oxide for Solid Oxide Fuel Cells, Ed. by T. Ishihara (Springer Science Business Media, LLC, 2009). https://doi.org/10.1007/978-0-387-77708-5
T. Ishihara, Encyclopedia of Applied Electrochemistry, Ed. by G. Kreysa, K. Ota, and R. F. Savinell (Springer, New York, 2014).https://doi.org/10.1007/978-1-4419-6996-5_165
N. Mahato, A. Banerjee, A. Gupta, et al., Prog. Mater. Sci. 72, 141 (2015). https://doi.org/10.1016/j.pmatsci.2015.01.001
D. Medvedev, A. Murashkina, E. Pikalova, et al., Prog. Mater. Sci. 60, 72 (2014). https://doi.org/10.1016/j.pmatsci.2013.08.001
S. Kato, M. Ogasawara, M. Sugai, et al., Solid State Ionics 149, 53 (2002). https://doi.org/10.1016/S0167-2738(02)00138-8
K. Fujii, M. Shiraiwa, and Y. Esaki, J. Mater. Chem. A 3, 11 985 (2015). https://doi.org/10.1039/C5TA01336D
X. Yang, S. Liu, F. Lu, et al., J. Phys. Chem. 120, 6416. https://doi.org/10.1021/acs.jpcc.6b01530
M. Shiraiwa, K. Fujii, Y. Esaki, et al., J. Electrochem. Soc. 164, F1392 (2017). https://doi.org/10.1149/2.0411713jes
Yu. A. Titov, N. M. Belyavina, and V. Ya. Markiv, Rep. Nat. Acad. Sci. Ukraine 10, 160 (1999).
N. Tarasova, I. Animitsa, A. Galisheva, et al., Materials 12, 1668 (2019). https://doi.org/10.3390/ma12101668
G. R. Ren, J. Y. Zhu, L. Li, et al., J. Am. Ceram. Soc. 100, 2582 (2017). https://doi.org/10.1111/jace.14644
J. C. Burley, P. D. Battle, P. Gaskell, et al., J. Solid State Chem. 168, 202 (2002). https://doi.org/10.1006/jssc.2002.9710
R. D. Shannon, Acta Crystallogr., Sect. A 32, 751 (1976). https://doi.org/10.1107/S0567739476001551
F. A. Kröger and H. J. Vink, Solid State Phys. 3, 307 (1956). https://doi.org/S0081-1947(08)60135-6
N. Tarasova, I. Animitsa, and A. Galisheva, J. Solid State Electrochem. 24, 1497 (2020). https://doi.org/10.1007/s10008-020-04630-1
Funding
This study was supported by the Ministry of Education and Science of the Russian Federation (State Assignment no. AAAA-A20-120061990010-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Tarasova, N.A., Animitsa, I.E., Galisheva, A.O. et al. Structure and Electrical Properties of a New Zn-Substituted Oxygen Ion Conductor Based on BaLaInO4 . Russ. J. Inorg. Chem. 66, 108–112 (2021). https://doi.org/10.1134/S0036023621010095
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621010095