Abstract—
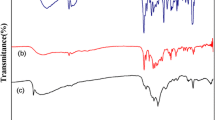
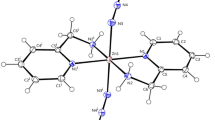
Cadmium sulfide (CdS) nanoparticles have been synthesized from CdX2 (X = Cl–, I–) complexes of 4-chlorobenzaldehyde and benzophenone thiosemicarbazone. The complexes have been studied by thermogravimetric analysis (TGA), elemental analysis, FT-IR, and NMR spectroscopy. The solvothermal decomposition of the complexes has afforded oleylamine-capped CdS nanoparticles with a wurtzite hexagonal phase. The transmission electron microscopy (TEM) studies show different morphologies which are influenced by reaction temperature and the nature of the ligands on the precursor complexes. Particles in the form of irregular cubes, elongated cubes and nanodendrites have been observed. The UV-Vis spectroscopy measurements show temperature and nature of complex-dependant optical properties. Blue-shifted band gap energies have been observed at lower reaction temperatures.








Similar content being viewed by others
REFERENCES
D.-S. Chuu and C.-M. Dai, Phys. Rev. B 45, 11 805 (1992).
Y. Guo, J. Wang, Z. Tao, et al., Cryst. Eng. Commun. 14, 1185 (2012).
F. H. Zhao, Q. Su, N. S. Xu, et al., J. Mater. Sci. 41, 1449 (2006).
J. S. Son, K. Park, S. G. Kwon, et al., Small 8, 2394 (2012).
G. Tai, W. Guo, Ultrason. Sonochem. 15, 350 (2008).
S. Dowland, T. Lutz, A. Ward, et al., Adv. Mater. 23, 2739 (2011).
H. Wang, Z. Sun, Q. Lu, F. Zeng, and D. Su, Small 8, 1167 (2012).
M. Kowshik, N. Deshmukh, W. Vogel, et al., Biotechnol. Bioeng. 78, 583 (2002).
M. Antoniadou and P. Lianos, J. Photochem. Photobiol. A: Chem. 204, 69 (2009).
G. B. Shombe, E. B. Mubofu, S. Mlowe, et al., Mater. Sci. Semicond. Process. 43, 230 (2016).
I. Pujalté, I. Passagne, B. Brouillaud, et al., Part Fibre Toxicol. 8, 10 (2011).
M. N. Rhyner, A. M. Smith, X. Gao, et al., Nanomedicine 1, 209 (2006).
C.-Y. Lai, B. G. Trewyn, D. M. Jeftinija, et al., J. Am. Chem. Soc. 125, 4451 (2003).
P. S. Nair, T. Radhakrishnan, N. Revaprasadu, et al., J. Mater. Chem. 12, 2722 (2002).
B. S. Rao, B. R. Kumar, V. R. Reddy, et al., Chalcogenide Lett. 8, 177 (2011).
S. Karan, B. Mallik, J. Phys. Chem. C 111, 16 734 (2007).
Z.-X. Cai, H. Yang, Y. Zhang, and X.-P. Yan, Anal. Chim. Acta 559, 234 (2006).
Y. Xia, Y. Xiong, B. Lim, and S. E. Skrabalak, Angew. Chem. Int. Ed. 48, 60 (2009).
M. Green, J. Mater. Chem. 20, 5797(2010).
S. Iravani, Green Chem. 13, 2638 (2011).
L. D. Nyamen, N. Revaprasadu, P. T. Ndifon, Mater. Sci. Semicond. Proc. 27, 191 (2014).
J. W. Kyobe, E. B. Mubofu, Y. M. M. Makame, et al., Physica E 76, 95 (2016).
S. Mlowe, R. Pullabhotla, E. Mubofu, et al., Int. Nano. Lett. 4, 106 (2014).
D. Fan, M. Afzaal, M. A. Malik, et al., Coord. Chem. Rev. 251, 1878 (2007).
L. D. Nyamen, V. S. R. Pullabhotla, A. A. Nejo, et al., New J. Chem. 35, 1133 (2011).
S. Mlowe, D. J. Lewis, M. A. Malik, et al., New J. Chem. 38, 6073 (2014).
V. S. R. Pullabhotla, M. Scriba, N. Revaprasadu, et al., Nanotechnol. 11, 1201 (2011).
N. Pradhan, B. Katz, and S. Efrima, J. Phys. Chem. B 107, 13 843 (2003).
A. L. Abdelhady, M. A. Malik, and P. O’Brien, J. Inorg. Organomet. Polym. Mater. 24, 226 (2014).
J. C. Bruce, N. Revaprasadu, and K. R. Koch, New J. Chem. 31, 1647 (2007).
C. Byrom, M. A. Malik, P. O’Brien, et al., Polyhedron 19, 211 (2000).
P. D. McNaughter, S. A. Saah, M. Akhtar, et al., Dalton Trans. 45, 16 345 (2016).
A. S. Pawar, S. C. Masikane, S. Mlowe, et al., Eur. J. Inorg. Chem. 366 (2016).
F. E. Anderson, C. J. Duca, and J. V. Scudi, J. Am. Chem. Soc. 73, 4967 (1951).
M. R. Kim, K. Miszta, M. Povia, et al., ACS Nano 6, 11 088 (2012).
J. Zhang, J. Gao, E. M. Miller, et al., ACS Nano 8, 614 (2014).
ACKNOWLEDGMENTS
The authors also thank the Microscopy and Microanalysis Unit (MMU) of the University of KwaZulu-Natal and Council for Scientific and Industrial Research, South Africa for transmission electron microscopy imaging.
Funding
The authors are grateful to the National Research Foundation (NRF, Grant number: 64820), South Africa, the India–Brazil–South Africa (IBSA) program, Royal Society-DFID program and the Department of Science and Technology (DST)-PURSE, India, for financial support.
Author information
Authors and Affiliations
Corresponding author
Supplementary material
APPENDIX A
APPENDIX A
Supporting information includes Scheme S1 (synthesis of complexes), proposed chemical structures (Fig. S1), 1H and 13C NMR spectra (Figs. S2–S9), IR spectra (Figs. S10–S13), thermograms (Fig. S14), powder X-ray diffraction patterns (Figs. S15–S17), UV-Vis absorption spectra (Figs. S18–S20), and average particle size diameter (Tables S1, S2) for compounds 1–4.
Rights and permissions
About this article
Cite this article
Siphamandla C. Masikane, Mlowe, S., Pawar, A.S. et al. Cadmium Chloride and Cadmium Iodide Thiosemicarbazone Complexes as Single Source Precursors for CdS Nanoparticles. Russ. J. Inorg. Chem. 64, 1063–1071 (2019). https://doi.org/10.1134/S0036023619080072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619080072