Abstract
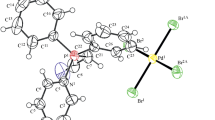
Ph3PC6H11-cyclo)]+[PdBr3(Dmso)]– (I) and [Ph3PBu]+[PdCl3(Dmso)]– (II) have been synthesized by the reactions of tetrahydrobromopalladic acid with (cyclohexyl)triphenylphosphonium bromide and tetrahydrochloropalladic acid with butyltriphenylphosphonium chloride in water with further recrystallization from dimethyl sulfoxide. [Ph3PCH2CH=CHCH2PPh3]2+[PdCl4]2–. Dmf (III) has been synthesized by a similar reaction of tetrahydrochloropalladic acid with butylene-2-bis(triphenylphosphonium) dichloride with recrystallization from N,N-dimethylformamide. According to X-ray diffraction data, P–C bond lengths and CPC angles in cations of complexes I–III variate within 1.778(2)–1.811(4) Å and 106.1(2)°–112.0(2)°, respectively. Dimethyl sulfoxide molecules in anions of complexes I and II are S-coordinated, and Pd–S bonds are 2.2478(14) and 2.2466(6) Å, while Pd–Cl distances in centrosymmetric square anions of complex III are 2.3143(5) and 2.3170(5) Å, and ClPdCl trans angles are 180°. The structural organization of crystals is formed by weak hydrogen bonds C–H...Br, C–H...Cl, and C–H...O.
Similar content being viewed by others
References
V. V. Sharutin, O. K. Sharutina, V. S. Senchurin, and I. A. Il’chenko, Russ. J. Coord. Chem. 41, 462 (2015).
V. V. Sharutin, V. S. Senchurin, and O. K. Sharutina, Russ. J. Inorg Chem. 58, 543 (2013).
V. V. Sharutin, V. S. Senchurin, O. K. Sharutina, and A. V. Gushchin, Butlerovskie Soobshch. 29, 26 (2012).
V. V. Sharutin, V. S. Senchurin, O. K. Sharutina, and A. V. Gushchin, Butlerovskie Soobshch. 30, 41 (2012).
SMART and SAINT-Plus. Data Collection and Processing Software for the SMART System (Bruker, Madison, Wisconsin, (1998), Version 5.0.
SHELXTL/PC. Ver. 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data Bruker, Madison, Wisconsin, (1998), version 5.10.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009).
V. V. Sharutin, V. S. Senchurin, and O. K. Sharutina, Butlerovskie Soobshch. 28, 31 (2011).
V. V. Sharutin, V. S. Senchurin, and O. K. Sharutina, Vestn. Yuzhno-Ural’sk. Gos. Univ., Ser. Khim., No. 33, 37 (2011).
S. S. Batsanov, Zh. Neorg. Khim. 36, 3015 (1991).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Sharutin, O.K. Sharutina, V.S. Senchurin, P.V. Andreev, 2018, published in Zhurnal Neorganicheskoi Khimii, 2018, Vol. 63, No. 6, pp. 712–717.
Rights and permissions
About this article
Cite this article
Sharutin, V.V., Sharutina, O.K., Senchurin, V.S. et al. Palladium Complexes [Ph3PC6H11-cyclo]+[PdBr3(Dmso-S)]–, [Ph3PBu]+[PdCl3(Dmso-S)]–, and [Ph3PCH2CH=CHCH2PPh3]2+[PdCl4]2–. Dmf: Synthesis and Structure. Russ. J. Inorg. Chem. 63, 747–752 (2018). https://doi.org/10.1134/S0036023618060220
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023618060220