Abstract
Cancer is a leading causes of death. Despite significant success in the treatment of lymphatic system tumors, the problems of relapse, drug resistance and effectiveness of therapy remain relevant. Oncolytic viruses are able to replicate in tumor cells and destroy them without affecting normal, healthy tissues. By activating antitumor immunity, viruses are effective against malignant neoplasms of various nature. In lymphoproliferative diseases with a drug-resistant phenotype, many cases of remissions have been described after viral therapy. The current level of understanding of viral biology and the discovery of host cell interaction mechanisms made it possible to create unique strains with high oncoselectivity widely used in clinical practice in recent years.
Similar content being viewed by others
INTRODUCTION
D.I. Ivanovsky’s discovery in 1892 of “filterable agents” that cause diseases [1], was the reason for a detailed study of the nature and properties of such infectious agents. In particular, it was found that some viruses are able to multiply effectively in the cells of malignant tumors, leading to the death of the latter.
At the beginning of the 20th century, a number of cases of spontaneous remission were described in patients with both hematological and solid tumors after vaccination or a viral infection. In 1904, a case of remission of lymphoma after suffering the flu was reported [2], and in 1912—after an emergency vaccination with a live anti-rabies vaccine—a remission of cervical cancer was observed [3]. Descriptions of such clinical cases served as prerequisites for the further study of viruses as antitumor agents.
The first documented descriptions of various viruses as oncolytic agents are given in Table 1.
Research on oncolytic viruses in Russia is associated with Professor Marina Konstantinovna Voroshilova. Under her leadership, the first strains of non-pathogenic enteroviruses (live enterovirus vaccines) were obtained, and in the 1970s, their unique oncolytic properties were revealed at the Institute of Poliomyelitis and Viral Encephalitis of the USSR Academy of Medical Sciences [11, 12]. Under the leadership of A.Ya. Muceniece, the antitumor properties of viruses have been analyzed in hundreds of patients with end-stage tumors of various histogenesis. One of the studied viral strains, ECHO-7, which underwent subsequent adaptation on tumor cells by bioselection, was approved for the treatment of melanoma under the name RIGVIR® and is widely used in clinical practice in a number of countries. [13].
In studies conducted in the second half of the 20th century, various methods of virus delivery were used, depending on the location of the tumor and the extent of the tumor process. Combinations of radical surgical treatment followed by the introduction of a viral preparation were also considered as promising strategies. However, insufficient understanding of the mechanisms of the oncolytic action of viruses and the presence of cases with severe side effects limited the possibility of developing new therapeutic regimens [14].
Modern methods of genetic engineering make it possible to increase the tumor specificity of viruses by enhancing their replication activity in tumor cells, as well as to modulate their immunogenic properties. At present, the development and study of new strains of oncolytic viruses is experiencing rapid growth [15]. Some have already been approved for use in clinical practice and are in use as anticancer drugs 13, 16‒19].
MECHANISMS OF VIRAL ONCOLYSIS
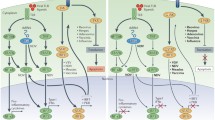
Oncolytic viruses are able to selectively destroy tumor cells through two main mechanisms: 1) by direct oncolysis (lysis of infected tumor cells) and 2) by inducing immune-mediated death of tumor cells through increased immune responses, which form, among other things, antitumor immunity. In addition, oncolytic viruses can induce the death of cells resistant to apoptosis, as well as resistant to existing anticancer drugs [15]. The result of direct oncolysis is the lysis of tumor cells at the stage of the next iteration of the virus life cycle, when the cell’s resources are completely depleted and it can no longer serve as a “factory” of viral particles. Factors that determine the selectivity of virus infection of tumor cells include the absence of a clear architecture of the tumor tissue, the presence of blood vessels with increased permeability [20], disturbances in the cellular antiviral response system, increased expression of molecules that serve as receptors for the penetration of the virus into the cell [21], and others. Due to the natural features of tumor cells, they become the preferred hosts for viruses in the body, in comparison with normal cells, as they effectively carry out the infectious cycle and spread of virions in the intercellular matrix. Penetration of virus into the cell can be mediated by various mechanisms of endocytosis [22, 23]. Receptor-mediated endocytosis is considered to be one of the key factors determining the efficiency of infection and tissue tropism of viral particles in general. To date, a wealth of information has been accumulated on the receptors and accessory molecules necessary for the successful spread of a viral infection24‒27]. In some cases, there is a correlation between the representation of the viral receptor on the cell surface and the efficiency of viral replication [28, 29]. Genetic engineering techniques, including genome editing systems such as TALEN and CRISPR/Cas9, allow the identification of previously unknown viral receptors and other key molecules required to initiate the infectious cycle.
One of the key mechanisms of antiviral protection at the cellular level is the induction of the interferon system [30], as a result of the activation of which the cell acquires a state of antiviral protection: the processes of transcription and translation are inhibited in order to prevent the production of viral components, interferon-β is secreted into the intercellular space, which serves as a “warning signal” for neighboring cells. Interferon secreted by an infected cell interacts with its receptors on the surface of uninfected cells, thereby activating the antiviral defense system in them [31]. In such a state, when the synthetic apparatus of the cell is almost non-functional, the cell loses its ability to divide. Tumor cells whose strategy involves uncontrolled division during malignant transformation, as a rule, lose the mechanisms that can stop/prevent the proliferation process. Thus, the accumulation of mutations in the genome of tumor cells leads to the loss of antiviral defense mechanisms. Cells that have lost sensitivity to interferon become an easy target for viruses and a favorable environment for further spread. [32].
Factors that increase the sensitivity of cells to viruses and facilitate virus-mediated oncolysis include mutations in the Ras/ERK, (PI3K)/Akt, and MAPK/ERK signaling pathway proteins. [33, 34], as well as increased expression of HIF-1α [35]. TP53 and PTEN gene mutations are also among the most common disorders [36‒40]. Changes in various signaling pathways that create favorable conditions for virus replication exclusively in tumor cells can occur at the epigenetic level [41].
STUDYING VIRAL ONCOLYSIS ON MODELS
IN VITRO AND IN VIVO
At the beginning of 20th century, the antitumor properties of viruses were studied exclusively on models in vivo—on spontaneously arising tumors of rodents. In 1922, Levaditi and Nicolau [42] first described the oncolytic properties of the vaccinia virus in various rat and mouse tumor models. Since the second half of the 20th century, the method of culturing mammalian cells has been actively used to study biological mechanisms in vitro. Cultures of the cell lines L929 and HeLa, obtained in 1948 and 1951, respectively, became the first models of viral oncolysis in vitro [43, 44].
In 1954, it was discovered that preliminary passaging of the encephalitis virus in vitro with subsequent intratumoral inoculation leads to a decrease in the proportion of mice with encephalitis, and also significantly reduces the ability of the virus to replicate in normal tissues [45]. Subsequently, this approach formed the basis of the method of bioselection of viruses with tropism for tumor cells. In the 1950s and 1960s, a number of studies were carried out in which the following was found: 1) The oncolytic effect of the Newcastle disease virus and influenza virus on the model of Ehrlich’s ascitic carcinoma [46‒48], 2) the oncolytic effect of various enteroviruses (poliovirus, coxsackieviruses of groups A and B) on models of immunodeficient mice with xenografts of tumor cells [49].
In animal models of lymphocytic leukemia (L4946) and Shaye’s leukemia, a positive effect on overall survival on the introduction of bovine enterovirus was revealed [50], and on the introduction of UV-inactivated and/or live vaccinia virus [51].
Thanks to the development of cell technologies, it became possible to maintain the viability of primary cultures of lymphoid tumors in vitro. Model lines of lymphoid tumors were first obtained in 1963 from Nigerian patients with Burkitt’s lymphoma: RAJI, JIJOYE, OGUN, and KUDI [52]. By the end of the 20th century, a number of different cultures of T- and B-cell LPD were obtained: cultures of Burkitt’s lymphoma EB-1, -2, -3 (from which Epstein–Barr virus was subsequently isolated) [53], cultures of T-cell acute lymphoblastic leukemia MOLT-1 and MOLT-5 [54], myelocytic cell line KG-1 [55] and dozens of others [56‒61]. The best characterized model lines, including Namalwa, Raji, U-937, and Jurkat, are deposited in the American Culture Collection (ATCC). A number of oncolytic strains have been successfully tested on these cultures. [62‒65].
Despite the possibility of obtaining stable cell lines, primary cultures of tumor cells, as a rule, serve as more reliable models for research [66, 67]. F. Babaeva et al. [68] studied the oncolytic activity of non-pathogenic enteroviruses on primary cell cultures of various B-cell LPDs.
Primary cultures of lymphomas are three-dimensional formations, to maintain the viability of which the method of cell cultivation in spheroids is used [69, 70]. This model close to physiological conditions— after all the tumor is, in fact, always a three-dimensional object. The interaction of the virus with a conglomerate of cells occurs differently than with cells in two-dimensional cultures that are in a monolayer, as in the 3D format, as well as in a tumor in situ not all cells of the conglomerate are available for infection at the initial stage of infection. But there are a number of additional factors that facilitate the spread of a viral infection and affect the efficiency of virus reproduction. Spheroids of tumor cells produce a large amount of cathepsins (like some tumors), which facilitates the penetration of viral particles into uninfected cells in a way that does not depend on canonical receptors. For example, cells of the U118-MG line with a “turned off” JAM-A receptor remain sensitive to reovirus infection while in spheroids, while in a monolayer this culture is resistant to reovirus infection [26].
ONCOLYTIC VIRUSES IN THE THERAPY OF LYMPHOPROLIFERATIVE DISEASES
Cases of spontaneous remissions of malignant diseases that occur after vaccination or transmission of a viral infection created fertile ground for research aimed at studying the oncolytic properties of various members of viral families.
In 1971, two cases of spontaneous regression of lymphoid tumors were described in Ugandans after measles infection. [71]:
— Burkitt’s lymphoma with lesions of the facial skeleton completely regressed within a week (a large tumor on the face rapidly decreased in size until it completely disappeared);
— complete disappearance of clinical symptoms in a patient with Hodgkin’s lymphoma (there was a decrease in lymph nodes, regression of B-symptoms).
A temporary but significant improvement in the clinical picture was observed after introduction of Langat viruses and Kyasanur forest disease in the fight against leukemia and other malignant neoplasms [72].
Measles Virus
In the 1970s, a number of cases of spontaneous remission of Hodgkin’s lymphoma in children after measles infection were described [71, 73‒76]. A significant improvement in the condition was observed in the case of acute lymphoblastic leukemia with a relapse aggravated by chemoresistance [77].
The measles virus is an RNA virus belonging to the Paramyxoviridae family, the genome of which includes one RNA molecule of negative polarity. When a virus enters a cell, CD150 [78], CD46 [79, 80] and nectin-4 [81, 82] molecules serve as receptors,. Overexpression of CD46 and nectin-4 molecules on tumor cells is a factor in the additional selectivity of the measles virus against malignant cells [83, 84]. With the help of genetic engineering, it is possible to modify the hemagglutinin of the measles virus in order to expand the tropism of the virus to tumor cells [85‒87] or to protect the virus from elimination by neutralizing antibodies [88].
Due to their high safety and genetic stability, vaccine strains of the measles virus are investigated as oncolytic agents [89]. Promising results have been obtained in an open Swiss study in patients with stage IIb cutaneous T-cell lymphoma who were resistant to conventional therapy [90]. Despite local therapy— intratumoral injections of a vaccine strain of the measles virus (Edmonton-Zagreb),—in some cases, regression of distant foci was observed. Side effects were minimal, indicating the safety of the use of this strain.
To date, the measles virus is considered a promising oncolytic agent, the therapeutic efficacy of which has been demonstrated both in animal models and in clinical trials involving cancer patients [91‒95], including those with multiple myeloma [96, 97].
Reovirus
Reoviruses are non-enveloped, spherically shaped viruses belonging to the Reoviridae family of viruses, the genome of which is represented by a segmented double-stranded RNA molecule.
REOLYSIN® (Oncolytic Biotech, Canada), also known under the trade name Pelareorep, was registered in 2013. It is an unmodified type 3 reovirus with a broad tropism for tumor cells, like other human orthoreoviruses [98]. The drug is widely used in the treatment of various cancers. Phase III clinical trials of combined treatment of head and neck tumors according to the scheme paclitaxel + carboplatin + reolysin have been successfully completed [99].
With the systemic administration of reovirus, the phenomenon of an antibody-dependent increase in infection is observed, which consists of the formation of the “reovirus-antibody” complex, its internalization by the cells of the immune system, and subsequent replication of the virus. Due to this mechanism, the cytotoxic effect of reovirus when administered intravenously not only does not decrease, but, on the contrary, increases. [100].
The study of the oncolytic activity of reovirus against multiple myeloma cells showed that its replication is more efficient in samples with resistance to bortezomib, a drug widely used in myeloma chemotherapy. Model cell lines and primary cultures obtained from patients with a refractory course of the disease were highly sensitive to reovirus with increased expression of the adhesion molecule JAM-A, which is typical for tumor cells that are not susceptible to bortezomib [101]. When studying the mechanisms of the oncolytic action of reovirus, a relationship was revealed between the activation of the Ras signaling pathway, the expression of cathepsins B, and the effectiveness of viral oncolysis [102, 103].
Vesicular Stomatitis Virus
The vesicular stomatitis virus (VSV) is part of the Rhabdoviridae family, its genome is a single-stranded RNA molecule of negative polarity. LDL-R (Low Density Lipoprotein Receptor) is the cellular receptor for VSV [104]. The reasons for considering VSV as a promising oncolytic agent are as follows: (1) low pathogenicity; (2) a wide tropism of the virus to cells of various origins, which is due to the wide receptor specificity of glycoprotein G; (3) high sensitivity of the virus to interferon (VSV predominantly replicates in cells with an impaired system of interferon induction and interferon response, which is typical for some tumor cells [105]); (4) the short 4-hour replication cycle contributes to the rapid death of tumor cells by the mechanism of direct oncolysis [106]. T-lymphocytes can be used as a cell carrier for delivery of VSV to the focus of tumor growth [107]. In addition, VSV can be used as a vector expressing immunomodulatory proteins [108].
A promising area of virotherapy is the treatment of acute myeloid leukemia (AML). For patients with recurrent AML, the range of therapeutic options is limited. In a mouse model in vivo a combined approach using viro- and immunotherapy had the best effect [109].
To predict the effectiveness of virotherapy, an analysis of molecular genetic determinants of the sensitivity of tumor cells is used [110].
Vaccinia Virus
The vaccinia virus developed to fight smallpox [111], has the longest and most extensive history of study compared to other viruses. The oncolytic properties of the vaccinia virus have also been studied.
The original smallpox virus belongs to the largest DNA virus family, the Poxviridae. More than 3 billion people have been vaccinated as part of the global smallpox eradication program, and clinical cases of spontaneous remissions of hematological malignancies have subsequently been described. Thus, a 78‑year-old man with chronic lymphocytic leukemia (CLL) remained in complete remission for 3 years after vaccination and relief of a severe local reaction and generalized rash with the introduction of human vaccinia globulin. In the clinical picture, all signs of CLL were absent and the number of leukocytes was within the normal range [112].
In recent years, various strains of the vaccinia virus have been studied as an oncolytic agent in various LPZs, in particular in in vivo and in vitro multiple myeloma models [113‒116].
The vaccinia virus has a pronounced tropism for tumor cells, and the large size of the viral particle makes it an attractive vector for delivering auxiliary therapeutic agents directly to the tumor. Thanks to genetic engineering, more and more oncolytic strains based on the vaccinia virus are being created, with the help of which it is possible to deliver various antitumor and/or immunostimulatory proteins, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) [117], apoptin [118], lactoferrin, and interleukin-15 [119], as well as antitumor miRNAs [120] and other molecules [116].
The oncolytic drug RGV004 is currently in clinical trials for the treatment of refractory/recurrent B-cell lymphoma (NCT04887025). This strain based on the vaccinia virus contains as a therapeutic agent a chimeric antibody of dual specificity to the CD19 and CD3 molecules [121]. The CD19 and CD3 antigens are components of multiprotein complexes on the surface of T- and B-lymphocytes, respectively. Thus, the predominant replication of the oncolytic strain in the tumor is achieved, and the therapeutic component, the anti-CD19/CD3 antibody, induces an additional immunostimulatory effect [122].
Newcastle Disease Virus
The Newcastle disease virus (NDV) is an RNA-containing avian virus from the Paramyxoviridae family. NDV causes a lethal infection in some species of birds, especially the order Galliformes, and is absolutely safe for humans [123]. Due to its high sensitivity to interferon, NDV replication efficiently occurs only in tumor cells [124], since the interferon induction system and the interferon response are disturbed.
The oncolytic activity of some NDV strains has been shown against various tumors in vitro, including cells of neuroblastoma, fibrosarcoma, colorectal and hepatocellular cancer, stomach and lung cancer, tumors of the mammary and prostate glands, and, importantly, glioblastoma [125]. Research in vitro and in vivo was also carried out in relation to LPD [126‒131], and in the middle of the 20th century NDV was used to treat acute leukemia [132].
With systemic administration of NDV in clinical trials involving patients with metastatic solid tumors (colorectal cancer, carcinomas of the pancreas, kidney, breast and non-small cell lung cancer), the following results were obtained: in phase I trials, improvement (complete, partial response or stabilization) was observed in 11.4% of cases [133]; in phase II, an increase in overall survival was registered [134]. In phase III clinical trials, which involved 567 patients, an increase in overall survival of patients with colon cancer was found [135].
Oncolytic Enteroviruses
Enteroviruses are non-enveloped viruses belonging to the Picornaviridae family, the genome of which is represented by a single-stranded RNA molecule of positive polarity.
In the 1950s, data appeared on the ability of some strains of enteroviruses to replicate in tumor cells, causing their death [50, 136]. The results of enteroviral cytolytic activity studies on models in vitro, in vivo, as well as for the treatment of malignant tumors in patients have been presented for echoviruses (ECHO) serotypes 1, 7 and 12 [11, 137‒139], poliovirus type 1 [140‒143] and Coxsackie A21 virus [144‒146]. Coxsackievirus A21 is registered under the trade name CAVATAK® and is used to treat melanoma, a non-invasive bladder cancer [16] and other malignant nosologies.
Several nonpathogenic strains from the ECHO and Coxsackie virus groups during the polio vaccination period were isolated from healthy children who did not develop full immunity against polio after vaccination. Asymptomatic enterovirus infection appears to prevent vaccine-strain poliovirus from colonizing the gut due to an interference effect. It was this effect that served as a stepping stone in the development of prophylactic live enterovirus vaccines (LEV), which were obtained at the Institute of Poliomyelitis and Viral Encephalitis of the USSR Academy of Medical Sciences in the 1960s and 1970s [147]. Two such vaccines based on the non-pathogenic ECHO-12 and ECHO-1 strains (ZEV-7 and ZhEV-4, respectively) have been tested in a large cohort of people during outbreaks of seasonal viral diseases and have been shown to be safe and effective [147, 148]. During the period of mass vaccination, a number of additional useful properties of LEVs were discovered, among which we note the following: clinical improvement in the condition of patients with herpes infections, multiple sclerosis, and oncological diseases. Over several years, a number of studies have been conducted, as a result of which the positive effect of LEV and vaccine strains of poliovirus on the course of oncological diseases with a resistant phenotype has been confirmed [149]. It has been established that enterovirus replication occurs in tumor cells, interferon induction is triggered, and T-cell antitumor immunity is stimulated. It has been shown that virotherapy can be combined with other treatments [150].
T-VEC—a Drug Based on Recombinant Herpes Virus
The purpose of the immune system is to recognize and remove foreign molecules from the body, which is also true for oncolytic viruses. That is why their action is limited to the period of formation of an adaptive immune response. Still, the immune system can act both as a barrier and as an ally in the fight against tumor cells.
Modulating immune system responses to minimize the antiviral response while promoting immune-mediated tumor cell lysis remains one of the key goals of virotherapy. Talimogene laherparepvec (T-VEC, ImlygicTM)—a viral drug approved by the US Food and Drug Administration (FDA) for the treatment of metastatic melanoma in 2013—is a recombinant strain of herpes simplex virus type 1 (HSV-1) [17, 18]. This is the first drug of “double” oncolytic action. It causes the death of tumor cells by the mechanism of direct oncolysis, as well as by suppressing immunosuppression and stimulating the immune system due to the production of GM-CSF by infected cells. The T-VEC preparation contains the GM-CSF nucleotide sequence in the expression cassette, and HSV-1 “serves” as a vector delivery system to tumor cells. This design provides expression of GM-CSF in tumors of patients within 48 hours [151]. In phase III clinical trials of the drug, it was found that local intratumoral injections of T-VEC in patients with advanced melanoma not only suppress tumor growth, but also, due to systemic action, prolong overall survival [18].
Other Viruses with Oncolytic Activity
The mumps virus is considered as a promising oncolytic agent in the treatment of gynecological malignancies [152].
Adenoviruses are among the best studied models for use both as vectors for vaccine development and as a model for viral oncolysis [153]. In 1996, a mutant strain ONYX-015 was constructed, in which the E1B-55K viral protein responsible for binding to the host cell p53 transcription factor and its inactivation was deleted [154]. It was assumed that the absence of this protein in the mutant adenovirus excludes the possibility of its replication in normal cells containing wild-type p53, but retains the ability to proliferate in tumor cells with mutant p53. Adenovirus H101, similar in its mechanism of action, was approved in November 2005 by the China Food and Drug Administration (SFDA, now CFDA) for cancer treatment, renamed Oncorine® (“Shanghai Sunway Biotech Co., Ltd, China) and is currently sold in the Chinese market [19].
During the COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), cases of spontaneous remission and regression of various LPDs have been reported: NK/T-cell lymphoma [155], primary cutaneous anaplastic large cell lymphoma [88], diffuse large B-cell lymphoma [156], acute myeloid leukemia and T-cell acute lymphoblastic leukemia [157],—as well as mycosis fungoides [158]. It is possible that the phenomenon of antibody-dependent increase in infection, which is also described for coronaviruses, also contributes to the regression of tumors of the lymphatic system against the background of SARS-CoV-2 infection [159]. As part of this process, massive death of immune cells carrying the FcRIIγ receptor (CD32), including monocytes and other cells of the immune system, can occur. At the moment, the literature does not provide information on the regression of tumors during the COVID-19 pandemic.
CONCLUSIONS
The natural oncolytic properties of viruses were discovered more than a century ago, and progress in understanding the molecular mechanisms of malignant cell transformation and the biology of viruses has led to the development of new effective and highly selective oncolytic strains.
Clinical trials have already demonstrated the safety and good tolerability of a number of oncolytic viruses, as well as their effectiveness both as drugs for monotherapy and in combination with traditional regimens of surgical treatment, radiation, chemotherapy and immunotherapy [99, 160‒162]. In addition, virotherapy can be considered as a promising approach in the fight against recurrent chemoresistant tumors [90, 101, 163]. A more detailed study of the mechanisms of viral oncolysis will make it possible to find and create new strains and therapeutic regimens for the treatment of resistant and recurrent lymphoproliferative diseases.
REFERENCES
Ivanowski D. 1892 Ueber die mosaikkrankheit der tabakspflanze. St. Petersb. Acad. Imp. Sci. Bul. 35, 67‒70.
Dock G. 1904. The influence of complicating diseases upon leukemia. Am. J. Med. Sci. 127, 563.
De Pace N. 1912. Sulla scomparsa di un enome canco vegetante del collo dell’utero senza cura chirurgica. Ginecologia. 9, 82‒89.
Hoster H.A., Zanes R.P., Jr., Von Haam E. 1949. Studies in Hodgkin’s syndrome; the association of viral hepatitis and Hodgkin’s disease; a preliminary report. Cancer Res. 9, 473‒480.
Higgins G.K., Pack G.T. 1951. Virus therapy in the treatment of tumors. Bull. Hosp. Joint Dis. 12, 379‒382.
Koprowska I. 1953. Morphologic changes of exfoliated cells in effusions of cancer patients following induced viral infections; preliminary observations. Am. J. Pathol. 29, 1105‒1121.
Moore A.E. 1954. Effects of viruses on tumors. Annu. Rev. Microbiol. 8, 393‒410.
Huebner R.J., Bell J.A., Rowe W.P., Ward T.G., Suskind R.G., Hartley J.W., Paffenbarger R.S., Jr. 1955. Studies of adenoidal-pharyngeal-conjunctival vaccines in volunteers. J. Am. Med. Assoc. 159, 986‒989.
Huebner R.J., Rowe W.P., Schatten W.E., Smith R.R., Thomas L.B. 1956. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 9, 1211‒1218.
Okuno Y., Asada T., Yamanishi K., Otsuka T., Takahashi M., Tanioka T., Aoyama H., Fukui O., Matsumoto K., Uemura F., Wada A. 1978. Studies on the use of mumps virus for treatment of human cancer. Biken J. 21, 37‒49.
Voroshilova M.K. 1988. Virological and immunological aspects of the use of LEV in oncological diseases. In Poleznye dlya ogranizma nepatogennye shtammy enterovirusov: Profilakticheskoe i lechebnoe ikh primenenie (Non-Pathogenic Strains of Enteroviruses Useful for the Body: Preventive and Therapeutic Use). Moscow: Izd. Minzdrava SSSR, 24‒29.
Voroshilova M.K., Tol’skaya E.A., Koroleva G.A., Chumakov K.M., Chumakov P.M. 1970. Study of the biological and morphological properties of the ECHO-I and ECHO-12 viruses. In Enterovirusnye infektsii (Enteroviral Infections). Tr. IPVE Akad. Med. Nauk SSSR. Moscow. 269–274.
Babiker H.M., Riaz I.B., Husnain M., Borad M.J. 2017. Oncolytic virotherapy including RIGVIR and standard therapies in malignant melanoma. Oncolytic Virother. 6, 11‒18.
Newman W., Southam C.M. 1954. Virus treatment in advanced cancer; a pathological study of fifty-seven cases. Cancer. 7, 106‒118.
De Munck J., Binks A., McNeish I.A., Aerts J.L. 2017. Oncolytic virus-induced cell death and immunity: a match made in heaven? J. Leukoc. Biol. 102, 631‒643.
Annels N.E., Mansfield D., Arif M., Ballesteros-Merino C., Simpson G.R., Denyer M., Sandhu S.S., Melcher A.A., Harrington K.J., Davies B., Au G., Grose M., Bagwan I., Fox B., Vile R., Mostafid H., Shafren D., Pandha H.S. 2019. Phase I trial of an ICAM-1-targeted immunotherapeutic-Coxsackievirus A21 (CVA21) as an oncolytic agent against non muscle-invasive bladder cancer. Clin. Cancer Res. 25, 5818‒5831.
Dolgin E. 2015. Oncolytic viruses get a boost with first FDA-approval recommendation. Nat. Rev. Drug Discov. 14, 369‒371.
Schmidt C. 2011. Amgen spikes interest in live virus vaccines for hard-to-treat cancers. Nat. Biotechnol. 29, 295‒296.
Liang M. 2018. Oncorine, the world first oncolytic virus medicine and its update in China. Curr. Cancer Drug Targets. 18, 171‒176.
Tseng J.C., Granot T., DiGiacomo V., Levin B., Meruelo D. 2010. Enhanced specific delivery and targeting of oncolytic sindbis viral vectors by modulating vascular leakiness in tumor. Cancer Gene Ther. 17, 244‒255.
Matveeva O., Kochneva G., Netesov S., Onikienko S., Chumakov P. 2015. Mechanisms of oncolysis by paramyxovirus Sendai. Acta Naturae. 7, 6‒16.
Maginnis M.S. 2018. Virus-receptor interactions: the key to cellular invasion. J. Mol. Biol. 430, 2590‒2611.
Marsh M., Helenius A. 2006. Virus entry: open sesame. Cell. 124, 729‒740.
Zhao X., Zhang G., Liu S., Chen X., Peng R., Dai L., Qu X., Li S., Song H., Gao Z., Yuan P., Liu Z., Li C., Shang Z., Li Y., Zhang M., Qi J., Wang H., Du N., Wu Y., Bi Y., Gao S., Shi Y., Yan J., Zhang Y., Xie Z., Wei W., Gao G.F. 2019. Human neonatal Fc receptor is the cellular uncoating receptor for enterovirus B. Cell. 177, 1553‒1565.e1516.
Barrass S.V., Butcher S.J. 2020. Advances in high-throughput methods for the identification of virus receptors. Med. Microbiol. Immunol. 209, 309‒323.
Dautzenberg I.J., van den Wollenberg D.J., van den Hengel S.K., Limpens R.W., Barcena M., Koster A.J., Hoeben R.C. 2014. Mammalian orthoreovirus T3D infects U-118 MG cell spheroids independent of junction adhesion molecule-A. Gene Ther. 21, 609‒617.
Lipatova A.V., Le T.H., Sosnovtseva A.O., Babaeva F.E., Kochetkov D.V., Chumakov P.M. 2018. Relationship between cell receptors and tumor cell sensitivity to oncolytic enteroviruses. Bull. Exp. Biol. Med. 166, 58‒62.
Huang Y.Y., Yu Z., Lin S.F., Li S., Fong Y., Wong R.J. 2007. Nectin-1 is a marker of thyroid cancer sensitivity to herpes oncolytic therapy. J. Clin. Endocrinol. Metab. 92, 1965‒1970.
Friedman G.K., Langford C.P., Coleman J.M., Cassady K.A., Parker J.N., Markert J.M., Yancey Gillespie G. 2009. Engineered herpes simplex viruses efficiently infect and kill CD133+ human glioma xenograft cells that express CD111. J. Neurooncol. 95, 199‒209.
Geoffroy K., Bourgeois-Daigneault M.C. 2020. The pros and cons of interferons for oncolytic virotherapy. Cytokine Growth Factor Rev. 56, 49‒58.
Katze M.G., He Y., Gale M., Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2, 675‒687.
Wollmann G., Robek M.D., van den Pol A.N. 2007. Variable deficiencies in the interferon response enhance susceptibility to vesicular stomatitis virus oncolytic actions in glioblastoma cells but not in normal human glial cells. J. Virol. 81, 1479‒1491.
Jin K.T., Tao X.H., Fan Y.B., Wang S.B. 2021. Crosstalk between oncolytic viruses and autophagy in cancer therapy. Biomed. Pharmacother. 134, 110932.
Choi A.H., O’Leary M.P., Lu J., Kim S.I., Fong Y., Chen N.G. 2018. Endogenous akt activity promotes virus entry and predicts efficacy of novel chimeric orthopoxvirus in triple-negative breast cancer. Mol. Ther. Oncolytics. 9, 22‒29.
Chakrabarty R., Tran H., Selvaggi G., Hagerman A., Thompson B., Coffey M. 2015. The oncolytic virus, pelareorep, as a novel anticancer agent: a review. Invest. New Drugs. 33, 761‒774.
Lin L., Su Z., Lebedeva I.V., Gupta P., Boukerche H., Rai T., Barber G.N., Dent P., Sarkar D., Fisher P.B. 2006. Activation of Ras/Raf protects cells from melanoma differentiation-associated gene-5-induced apoptosis. Cell Death Differ. 13, 1982‒1993.
Noser J.A., Mael A.A., Sakuma R., Ohmine S., Marcato P., Lee P.W., Ikeda Y. 2007. The RAS/Raf1/ MEK/ERK signaling pathway facilitates VSV-mediated oncolysis: implication for the defective interferon response in cancer cells. Mol. Ther. 15, 1531‒1536.
Blackham A.U., Northrup S.A., Willingham M., Sirintrapun J., Russell G.B., Lyles D.S., Stewart J.H. 2014. Molecular determinants of susceptibility to oncolytic vesicular stomatitis virus in pancreatic adenocarcinoma. J. Surg. Res. 187, 412‒426.
Cascallo M., Capella G., Mazo A., Alemany R. 2003. Ras-dependent oncolysis with an adenovirus VAI mutant. Cancer Res. 63, 5544‒5550.
Pikor L.A., Bell J.C., Diallo J.-S. 2015. Oncolytic viruses: exploiting cancer’s deal with the devil. Trends Cancer. 1, 266‒277.
Li Q., Tainsky M.A. 2011. Epigenetic silencing of IRF7 and/or IRF5 in lung cancer cells leads to increased sensitivity to oncolytic viruses. PLoS One. 6, e28683.
Levaditi C., Nicolau S. 1922. Sur le culture du virus vaccinal dans les neoplasmes epithelieux. CR Soc. Biol. 86, 928.
Weller T.H., Robbins F.C., Enders J.F. 1949. Cultivation of poliomyelitis virus in cultures of human foreskin and embryonic tissues. Proc. Soc. Exp. Biol. Med. 72, 153‒155.
Grayston J.T., Johnston P.B., Loosli C.G., Smith M.E. 1956. An improved technique for the neutralization test with adenoviruses in HeLa cell cultures. J. Infect. Dis. 99, 188‒198.
Moore A.E. 1951. Inhibition of growth of five transplantable mouse tumors by the virus of Russian Far East encephalitis. Cancer. 4, 375‒382.
Cassel W.A. 1957. Multiplication of influenza virus in the Ehrlich ascites carcinoma. Cancer Res. 17, 618‒622.
Flanagan A.D., Love R., Tesar W. 1955. Propagation of Newcastle disease virus in Ehrlich ascites cells in vitro and in vivo. Proc. Soc. Exp. Biol. Med. 90, 82‒86.
Nemunaitis J. 1999. Oncolytic viruses. Invest. New Drugs. 17, 375‒386.
Suskind R.G., Huebner R.J., Rowe W.P., Love R. 1957. Viral agents oncolytic for human tumors in heterologous host; oncolytic effect of Coxsackie B viruses. Proc. Soc. Exp. Biol. Med. 94, 309‒318.
Taylor M.W., Cordell B., Souhrada M., Prather S. 1971. Viruses as an aid to cancer therapy: regression of solid and ascites tumors in rodents after treatment with bovine enterovirus. Proc. Natl. Acad. Sci. U. S. A . 68, 836‒840.
Zakay-Roness Z., Bernkopf H. 1964. Effect of active and ultraviolet-irradiated inactive vaccinia virus on the development of Shay leukemia in rats. Cancer Res. 24, 373‒378.
Pulvertaft J.V. 1964. Cytology of Burkitt’s tumour (African lymphoma). Lancet. 1, 238‒240.
Epstein M.A., Barr Y.M. 1965. Characteristics and mode of growth of tissue culture strain (Eb1) of human lymphoblasts from Burkitt’s lymphoma. J. Natl. Cancer Inst. 34, 231‒240.
Minowada J., Onuma T., Moore G.E. 1972. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J. Natl. Cancer Inst. 49, 891‒895.
Koeffler H.P., Golde D.W. 1978. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 200, 1153‒1154.
Lozzio B.B., Lozzio C.B. 1979. Properties and usefulness of the original K-562 human myelogenous leukemia cell line. Leuk. Res. 3, 363‒370.
Sundstrom C., Nilsson K. 1976. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer. 17, 565‒577.
Schneider U., Schwenk H.U., Bornkamm G. 1977. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int. J. Cancer. 19, 621‒626.
Hurwitz R., Hozier J., LeBien T., Minowada J., Gajl-Peczalska K., Kubonishi I., Kersey J. 1979. Characterization of a leukemic cell line of the pre-B phenotype. Int. J. Cancer. 23, 174‒180.
Drexler H.G., Minowada J. 1998. History and classification of human leukemia-lymphoma cell lines. Leuk. Lymphoma. 31, 305‒316.
Drexler H.G., MacLeod R.A. 2003. Leukemia-lymphoma cell lines as model systems for hematopoietic research. Ann. Med. 35, 404‒412.
Maurer S., Salih H.R., Smirnow I., Lauer U.M., Berchtold S. 2019. Suicide gene‑armed measles vaccine virus for the treatment of AML. Int. J. Oncol. 55, 347‒358.
Hall K., Scott K.J., Rose A., Desborough M., Harrington K., Pandha H., Parrish C., Vile R., Coffey M., Bowen D. 2012. Reovirus-mediated cytotoxicity and enhancement of innate immune responses against acute myeloid leukemia. BioRes. Open Access. 1 (1), 3‒15.
Madlambayan G.J., Bartee E., Kim M., Rahman M.M., Meacham A., Scott E.W., McFadden G., Cogle C.R. 2012. Acute myeloid leukemia targeting by myxoma virus in vivo depends on cell binding but not permissiveness to infection in vitro. Leuk. Res. 36, 619‒624.
Koldehoff M., Lindemann M., Opalka B., Bauer S., Ross R.S., Elmaagacli A.H. 2015. Cytomegalovirus induces apoptosis in acute leukemia cells as a virus-versus-leukemia function. Leuk. Lymphoma. 56, 3189‒3197.
Drexler H.G., Pommerenke C., Eberth S., Nagel S. 2018. Hodgkin lymphoma cell lines: to separate the wheat from the chaff. Biol. Chem. 399, 511‒523.
Younes A., Drach J., Katz R., Jendiroba D., Sabourian M.H., Sarris A.H., Swan F., Jr., Hill D., Cabanillas F., Ford R., Andreeff M. 1994. Growth inhibition of follicular small-cleaved-cell lymphoma cells in short-term culture by interleukin-3. Ann. Oncol. 5, 265‒268.
Babaeva F., Lipatova A., Kochetkov D., Chumakov P., Kravchenko S. 2019. Study of reproduction of oncolytic viruses in organ cultures of human lymphoid tumors. Onkogematologiya. 14, 84–90.
Foxall R., Narang P., Glaysher B., Hub E., Teal E., Coles M.C., Ashton-Key M., Beers S.A., Cragg M.S. 2020. Developing a 3D B cell lymphoma culture system to model antibody therapy. Front. Immunol. 11, 605231.
Lamaison C., Latour S., Helaine N., Le Morvan V., Saint-Vanne J., Mahouche I., Monvoisin C., Dussert C., Andrique L., Deleurme L., Dessauge E., Pangault C., Baulande S., Legoix P., Seffals M., Broca-Brisson L., Alessandri K., Carlotti M., Soubeyran P., Merlio J.P., Mourcin F., Nassoy P., Recher G., Tarte K., Bresson-Bepoldin L. 2021. A novel 3D culture model recapitulates primary FL B-cell features and promotes their survival. Blood Adv. 5, 5372‒5386.
Bluming A.Z., Ziegler J.L. 1971. Regression of Burkitt’s lymphoma in association with measles infection. Lancet. 2, 105‒106.
Webb H.E., Wetherley-Mein G., Smith C.E., McMahon D. 1966. Leukaemia and neoplastic processes treated with Langat and Kyasanur forest disease viruses: a clinical and laboratory study of 28 patients. Br. Med. J. 1, 258‒266.
Pasquinucci G. 1971. Possible effect of measles on leukaemia. Lancet. 1, 136.
Zygiert Z. 1971. Hodgkin’s disease: remissions after measles. Lancet. 1, 593.
Mota H.C. 1973. Infantile hodgkin’s disease: remission after measles. Br. Med. J. 2, 421.
Taqi A.M., Abdurrahman M.B., Yakubu A.M., Fleming A.F. 1981. Regression of Hodgkin’s disease after measles. Lancet. 1, 1112.
Gross S. 1971. Measles and leukaemia. Lancet. 1, 397‒398.
Romanets-Korbut O., Kovalevska L.M., Seya T., Sidorenko S.P., Horvat B. 2016. Measles virus hemagglutinin triggers intracellular signaling in CD150-expressing dendritic cells and inhibits immune response. Cell Mol. Immunol. 13, 828‒838.
Naniche D., Varior-Krishnan G., Cervoni F., Wild T., Rossi B., Rabourdin-Combe C., Gerlier D. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67, 6025‒6032.
Anderson B.D., Nakamura T., Russell S.J., Peng K.W. 2004. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 64, 4919‒4926.
Dorig R., Marcil A., Chopra A. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain) cell. Cell. 75, 295–305.
Mateo M., Navaratnarajah C.K., Syed S., Cattaneo R. 2013. The measles virus hemagglutinin β-propeller head β4-β5 hydrophobic groove governs functional interactions with nectin-4 and CD46 but not those with the signaling lymphocytic activation molecule. J. Virol. 87, 9208‒9216.
Jurianz K., Ziegler S., Garcia-Schüler H., Kraus S., Bohana-Kashtan O., Fishelson Z., Kirschfink M. 1999. Complement resistance of tumor cells: basal and induced mechanisms. Mol. Immunol. 36, 929‒939.
Surowiak P., Materna V., Maciejczyk A., Kaplenko I., Spaczynski M., Dietel M., Lage H., Zabel M. 2006. CD46 expression is indicative of shorter revival-free survival for ovarian cancer patients. Anticancer Res. 26 (6C), 4943‒4948.
Allen C., Vongpunsawad S., Nakamura T., James C.D., Schroeder M., Cattaneo R., Giannini C., Krempski J., Peng K.-W., Goble J.M. 2006. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 66, 11840‒11850.
Munoz-Alia M.A., Nace R.A., Tischer A., Zhang L., Bah E.S., Auton M., Russell S.J. 2021. MeV-stealth: a CD46-specific oncolytic measles virus resistant to neutralization by measles-immune human serum. PLoS Pathog. 17, e1009283.
Msaouel P., Iankov I.D., Allen C., Russell S.J., Galanis E. 2012. Oncolytic measles virus retargeting by ligand display. Methods Mol. Biol. 797, 141‒162.
Gambichler T., Boms S., Hessam S., Tischoff I., Tannapfel A., Luttringhaus T., Beckman J., Stranzenbach R. 2021. Primary cutaneous anaplastic large-cell lymphoma with marked spontaneous regression of organ manifestation after SARS-CoV-2 vaccination. Br. J. Dermatol. 185, 1259‒1262.
Cutts F.T., Markowitz L.E. 1994. Successes and failures in measles control. J. Infect. Dis. 170, S32‒S41.
Heinzerling L., Kunzi V., Oberholzer P.A., Kundig T., Naim H., Dummer R. 2005. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 106, 2287‒2294.
Allen C., Paraskevakou G., Liu C., Iankov I.D., Msaouel P., Zollman P., Myers R., Peng K.W., Russell S.J., Galanis E. 2008. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin. Biol. Ther. 8 (2), 213‒220.
Galanis E., Hartmann L.C., Cliby W.A., Long H.J., Peethambaram P.P., Barrette B.A., Kaur J.S., Haluska P.J., Aderca I., Zollman P.J. 2010. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 70, 875‒882.
Iankov I.D., Msaouel P., Allen C., Federspiel M.J., Bulur P.A., Dietz A.B., Gastineau D., Ikeda Y., Ingle J.N., Russell S.J. 2010. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res. Treat. 122, 745‒754.
Liu C., Sarkaria J.N., Petell C.A., Paraskevakou G., Zollman P.J., Schroeder M., Carlson B., Decker P.A., Wu W., James C.D. 2007. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin. Cancer Res. 13, 7155‒7165.
Msaouel P., Iankov I.D., Allen C., Morris J.C., Von Messling V., Cattaneo R., Koutsilieris M., Russell S.J., Galanis E. 2009. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate. 69, 82‒91.
Dingli D., Peng K.W., Harvey M.E., Greipp P.R., O’Connor M.K., Cattaneo R., Morris J.C., Russell S.J. 2004. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 103, 1641‒1646.
Ong H.T., Timm M.M., Greipp P.R., Witzig T.E., Dispenzieri A., Russell S.J., Peng K.W. 2006. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp. Hematol. 34, 713‒720.
Muller L., Berkeley R., Barr T., Ilett E., Errington-Mais F. 2020. Past, present and future of oncolytic reovirus. Cancers (Basel). 12, 3219.
Carew J.S., Espitia C.M., Zhao W., Kelly K.R., Coffey M., Freeman J.W., Nawrocki S.T. 2013. Reolysin is a novel reovirus-based agent that induces endoplasmic reticular stress-mediated apoptosis in pancreatic cancer. Cell Death Dis. 4, e728.
Berkeley R.A., Steele L.P., Mulder A.A., van den Wollenberg D.J.M., Kottke T.J., Thompson J., Coffey M., Hoeben R.C., Vile R.G., Melcher A., Ilett E.J. 2018. Antibody-neutralized reovirus is effective in oncolytic virotherapy. Cancer Immunol. Res. 6, 1161‒1173.
Kelly K.R., Espitia C.M., Zhao W., Wendlandt E., Tricot G., Zhan F., Carew J.S., Nawrocki S.T. 2015. Junctional adhesion molecule-A is overexpressed in advanced multiple myeloma and determines response to oncolytic reovirus. Oncotarget. 6, 41275‒41289.
Strong J.E., Coffey M.C., Tang D., Sabinin P., Lee P.W. 1998. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17, 3351‒3362.
Phillips M.B., Stuart J.D., Rodriguez Stewart R.M., Berry J.T., Mainou B.A., Boehme K.W. 2018. Current understanding of reovirus oncolysis mechanisms. Oncolytic. Virother. 7, 53‒63.
Nikolic J., Belot L., Raux H., Legrand P., Gaudin Y., Albertini A.A. 2018. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat. Commun. 9, 1029.
Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991‒998.
Finkelshtein D., Werman A., Novick D., Barak S., Rubinstein M. 2013. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. U. S. A. 110, 7306‒7311.
Qiao J., Kottke T., Willmon C., Galivo F., Wongthida P., Diaz R.M., Thompson J., Ryno P., Barber G.N., Chester J., Selby P., Harrington K., Melcher A., Vile R.G. 2008. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat. Med. 14, 37‒44.
Lichty B.D., Power A.T., Stojdl D.F., Bell J.C. 2004. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol. Med. 10, 210‒216.
Shen W., Patnaik M.M., Ruiz A., Russell S.J., Peng K.W. 2016. Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia. Blood. 127, 1449‒1458.
Hastie E., Cataldi M., Marriott I., Grdzelishvili V.Z. 2013. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 176 (1‒2), 16‒32.
Jenner E. 1800. Dr. Jenner on the vaccine inoculation. Med. Phys. J. 3 (16), 502‒503.
Hansen R.M., Libnoch J.A. 1978. Remission of chronic lymphocytic leukemia after smallpox vaccination. Arch. Intern. Med. 138, 1137‒1138.
Lei W., Wang S., Xu N., Chen Y., Wu G., Zhang A., Chen X., Tong Y., Qian W. 2020. Enhancing therapeutic efficacy of oncolytic vaccinia virus armed with Beclin-1, an autophagic gene in leukemia and myeloma. Biomed. Pharmacother. 125, 110030.
Futami M., Sato K., Miyazaki K., Suzuki K., Nakamura T., Tojo A. 2017. Efficacy and safety of doubly-regulated vaccinia virus in a mouse xenograft model of multiple myeloma. Mol. Ther. Oncolytics. 6, 57‒68.
Deng H., Tang N., Stief A.E., Mehta N., Baig E., Head R., Sleep G., Yang X.Z., McKerlie C., Trudel S., Stewart A.K., McCart J.A. 2008. Oncolytic virotherapy for multiple myeloma using a tumour-specific double-deleted vaccinia virus. Leukemia. 22, 2261‒2264.
Guo Z.S., Lu B., Guo Z., Giehl E., Feist M., Dai E., Liu W., Storkus W.J., He Y., Liu Z., Bartlett D.L. 2019. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J. Immunother. Cancer. 7, 6.
Thorne S.H., Hwang T.H., O’Gorman W.E., Bartlett D.L., Sei S., Kanji F., Brown C., Werier J., Cho J.H., Lee D.E., Wang Y., Bell J., Kirn D.H. 2007. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J. Clin. Invest. 117, 3350‒3358.
Zhou S., Zhang M., Zhang J., Shen H., Tangsakar E., Wang J. 2012. Mechanisms of apoptin-induced cell death. Med. Oncol. 29, 2985‒2991.
Kowalsky S.J., Liu Z., Feist M., Berkey S.E., Ma C., Ravindranathan R., Dai E., Roy E.J., Guo Z.S., Bartlett D.L. 2018. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol. Ther. 26, 2476‒2486.
Lei W., Wang S., Yang C., Huang X., Chen Z., He W., Shen J., Liu X., Qian W. 2016. Combined expression of miR-34a and Smac mediated by oncolytic vaccinia virus synergistically promote anti-tumor effects in multiple myeloma. Sci. Rep. 6, 32174.
Topp M.S., Gokbuget N., Zugmaier G., Klappers P., Stelljes M., Neumann S., Viardot A., Marks R., Diedrich H., Faul C., Reichle A., Horst H.A., Bruggemann M., Wessiepe D., Holland C., Alekar S., Mergen N., Einsele H., Hoelzer D., Bargou R.C. 2014. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B‑precursor acute lymphoblastic leukemia. J. Clin. Oncol. 32, 4134‒4140.
Lei W., Ye Q., Hao Y., Chen J., Huang Y., Yang L., Wang S., Qian W. 2022. CD19-targeted bite expression by an oncolytic vaccinia virus significantly augments therapeutic efficacy against B-cell lymphoma. Blood Cancer J. 12, 35.
Yurchenko K.S., Zhou P., Kovner A.V., Zavjalov E.L., Shestopalova L.V., Shestopalov A.M. 2018. Oncolytic effect of wild-type Newcastle disease virus isolates in cancer cell lines in vitro and in vivo on xenograft model. PLoS One. 13, e0195425.
Zhang S., Sun Y., Chen H., Dai Y., Zhan Y., Yu S., Qiu X., Tan L., Song C., Ding C. 2014. Activation of the PKR/eIF2α signaling cascade inhibits replication of newcastle disease virus. Virol. J. 11, 62. https://doi.org/10.1186/1743-422X-11-62
Matveeva O.V., Kochneva G.V., Zaynutdinov S.S., Il’inskaya G.V., Chumakov P.M. 2018. Oncolytic paramyxoviruses: mechanism of action, preclinical and clinical studies. Mol. Biol. (Moscow). 52 (3), 306–322.
Cassel W.A., Garrett R.E. 1965. Newcastle disease virus as an antineoplastic agent. Cancer. 18, 863‒868.
Bar-Eli N., Giloh H., Schlesinger M., Zakay-Rones Z. 1996. Preferential cytotoxic effect of Newcastle disease virus on lymphoma cells. J. Cancer Res. Clin. Oncol. 122, 409‒415.
Eaton M.D., Almquist S.J. 1975. Antibody response of syngeneic mice to membrane antigens from NDV-lnfected lymphoma. Proc. Soc. Exp. Biol. Med. 148, 1090‒1094.
Eaton M.D., Levinthal J.D., Scala A.R. 1967. Contribution of antiviral immunity to oncolysis by Newcastle disease virus in a murine lymphoma. J. Natl. Cancer Inst. 39, 1089‒1097.
Al-Shammari A.M., Rameez H., Al-Taee M.F. 2016. Newcastle disease virus, rituximab, and doxorubicin combination as anti-hematological malignancy therapy. Oncolytic Virother. 5, 27‒34.
Klafuric E. 2022. Combining Newcastle disease virus and decitabine enhances leukemia cell death in models of murine acute T-cell lymphocytic and acute myeloid leukemias. Thesis of Master of Science in Pathobiology, University of Guelph, https://atrium.lib.uoguelph.ca/ xmlui/handle/10214/26655.
Wheelock E.F., Dingle J.H. 1964. Observations on the repeated administration of viruses to a patient with acute leukemia. A preliminary report. N. Engl. J. Med. 271, 645‒651.
Lorence R.M., Scot Roberts M., O’Neil J.D., Groene W.S., Miller J.A., Mueller S.N., Bamat M.K. 2007. Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle disease virus. Curr. Cancer Drug Targets. 7, 157‒167.
Csatary L., Eckhardt S., Bukosza I., Czegledi F., Fenyvesi C., Gergely P., Bodey B., Csatary C. 1993. Attenuated veterinary virus vaccine for the treatment of cancer. Cancer Detect. Prev. 17, 619‒627.
Liang W., Wang H., Sun T.-M., Yao W.-Q., Chen L.-L., Jin Y., Li C.-L., Meng F.-J. 2003. Application of auto-logous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive traet. World J. Gastroenterol. 9, 495‒498.
Au G.G., Beagley L.G., Haley E.S., Barry R.D., Shafren D.R. 2011. Oncolysis of malignant human melanoma tumors by Coxsackieviruses A13, A15 and A18. Virol. J. 8, 22.
Berry L.J., Au G.G., Barry R.D., Shafren D.R. 2008. Potent oncolytic activity of human enteroviruses against human prostate cancer. Prostate. 68, 577‒587.
Haley E.S., Au G.G., Carlton B.R., Barry R.D., Shafren D.R. 2009. Regional administration of oncolytic Echovirus 1 as a novel therapy for the peritoneal dissemination of gastric cancer. J. Mol. Med. 87, 385‒399.
Mutsenietse A.Ya. 1978. Study of the sensitivity of human melanomas to enteroviruses adapted to these tumors. In Virusy v terapii opukholei. (Viruses in Tumor Therapy). Riga: Zinatne, 175‒189.
Dobrikova E.Y., Broadt T., Poiley-Nelson J., Yang X., Soman G., Giardina S., Harris R., Gromeier M. 2008. Recombinant oncolytic poliovirus eliminates glioma in vivo without genetic adaptation to a pathogenic phenotype. Mol. Ther. 16, 1865‒1872.
Gromeier M., Lachmann S., Rosenfeld M.R., Gutin P.H., Wimmer E. 2000. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl. Acad. Sci. U. S. A . 97, 6803‒6808.
Toyoda H., Ido M., Hayashi T., Gabazza E.C., Suzuki K., Kisenge R.R., Kang J., Hori H., Komada Y. 2004. Experimental treatment of human neuroblastoma using live-attenuated poliovirus. Int. J. Oncol. 24, 49‒58.
Toyoda H., Wimmer E., Cello J. 2011. Oncolytic poliovirus therapy and immunization with poliovirus-infected cell lysate induces potent antitumor immunity against neuroblastoma in vivo. Int. J. Oncol. 38, 81‒87.
Au G.G., Lincz L.F., Enno A., Shafren D.R. 2007. Oncolytic Coxsackievirus A21 as a novel therapy for multiple myeloma. Br. J. Haematol. 137, 133‒141.
Au G.G., Lindberg A.M., Barry R.D., Shafren D.R. 2005. Oncolysis of vascular malignant human melanoma tumors by Coxsackievirus A21. Int. J. Oncol. 26, 1471‒1476.
Skelding K.A., Barry R.D., Shafren D.R. 2009. Systemic targeting of metastatic human breast tumor xenografts by Coxsackievirus A21. Breast Cancer Res. Treat. 113, 21‒30.
Chumakov M.P., Voroshilova M.K., Antsupova A.S., Boyko V.M., Blinova M.I., Prymyagi L.S., Rodin V.I., Seybilь V.B., Sinyak K.M., Smorodintsev A.A., Stepanchuk V.A., Terekhov S.N., Trofimova L.I., Chumakov P.M. 1992. Live enterovirus vaccines for emergency non-specific prevention of mass respiratory diseases during autumn-winter epidemics of influenza and acute respiratory diseases. Zh. Mikrobiol. Epidemiol. Immunobiol. 11–12, 37‒40.
Voroshilova M.K. 1970. Live enterovirus vaccines. Materialy 13 Vsesoyuznogo s”ezda epidemiologov, mikrobiologov i infektsionistov. (Proc. 13 All-Union Congr. Epidemiol., Microbiol., Infect.). Tbilisi, 355.
Voroshilova M.K. 1989. Potential use of nonpathogenic enteroviruses for control of human disease. Prog. Med. Virol. 36, 191‒202.
Voroshilova M.K., Goryunova A.G., Gorbachkova E.A., Chumakov P.M., Oganyan G.R., Kodkind G.X. 1977. Study of cellular immunity in cancer patients during asymptomatic enterovirus infection. In Viroterapiya i iskusstvennaya geterogenizatsiya opukholei (Virotherapy and Artificial Heterogenization of Tumors). Riga: Zinatne, 17‒19.
Hu J.C., Coffin R.S., Davis C.J., Graham N.J., Groves N., Guest P.J., Harrington K.J., James N.D., Love C.A., McNeish I., Medley L.C., Michael A., Nutting C.M., Pandha H.S., Shorrock C.A., Simpson J., Steiner J., Steven N.M., Wright D., Coombes R.C. 2006. A phase I study of OncovexGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 12, 6737‒6747.
Shimizu Y., Hasumi K., Okudaira Y., Yamanishi K., Takahashi M. 1988. Immunotherapy of advanced gynecologic cancer patients utilizing mumps virus. Cancer Detect. Prev. 12, 487‒495.
Stepanenko A.A., Chekhonin V.P. 2018. A compendium of adenovirus genetic modifications for enhanced replication, oncolysis, and tumor immunosurveillance in cancer therapy. Gene. 679, 11‒18.
Kirn D., Hermiston T., McCormick F. 1998. ONYX-015: clinical data are encouraging. Nat. Med. 4, 1341‒1342.
Pasin F., Mascalchi Calveri M., Calabrese A., Pizzarelli G., Bongiovanni I., Andreoli M., Cattaneo C., Rignanese G. 2020. Oncolytic effect of SARV-CoV2 in a patient with NK lymphoma. Acta Biomed. 91 (3), e2020047.
Paz A., Condori X., Hofmann A., Soares T., Predebon V., Siqueira V., Dortzbacher F., Calvache E., Gomes C., Portich J. 2021. SARS-CoV-2 induced remission of diffuse large B-cell lymphoma: a case report. Hematol. Transfusion Cell Therapy. 43 (S1), s103. https://doi.org/10.1016/j.htct.2021.10.175
Kandeel E.Z., Refaat L., Abdel-Fatah R., Samra M., Bayoumi A., Abdellateif M.S., Abdel-Hady H., Ali M., Khafagy M. 2021. Could COVID-19 induce remission of acute leukemia? Hematology. 26, 870‒873.
Ohadi L., Hosseinzadeh F., Dadkhahfar S., Nasiri S. 2022. Oncolytic effect of SARS-CoV-2 in a patient with mycosis fungoides (MF): a case repor Clin. Case Rep. 10 (4), e05682.
Nechipurenko Y.D., Anashkina A.A., Matveeva O.V. 2020. Change of antigenic determinants of SARS-CoV-2 virus S-protein as a possible cause of antibody-dependent enhancement of virus infection and cytokine storm. Biophysics (Oxford). 65, 703‒709.
Markert J.M., Liechty P.G., Wang W., Gaston S., Braz E., Karrasch M., Nabors L.B., Markiewicz M., Lakeman A.D., Palmer C.A., Parker J.N., Whitley R.J., Gillespie G.Y. 2009. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol. Ther. 17, 199‒207.
Sun L., Funchain P., Song J.M., Rayman P., Tannenbaum C., Ko J., McNamara M., Marcela Diaz-Montero C., Gastman B. 2018. Talimogene laherparepvec combined with anti-PD-1 based immunotherapy for unresectable stage III‒IV melanoma: a case series. J. Immunother. Cancer. 6, 36.
Mell L.K., Brumund K.T., Daniels G.A., Advani S.J., Zakeri K., Wright M.E., Onyeama S.J., Weisman R.A., Sanghvi P.R., Martin P.J., Szalay A.A. 2017. Phase I trial of intravenous oncolytic vaccinia virus (GL-ONC1) with cisplatin and radiotherapy in patients with locoregionally advanced head and neck carcinoma. Clin. Cancer Res. 23, 5696‒5702.
Beasley G.M., Nair S.K., Farrow N.E., Landa K., Selim M.A., Wiggs C.A., Jung S.H., Bigner D.D., True Kelly A., Gromeier M., Salama A.K. 2021. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J. Immunother. Cancer. 9, e002203.
Funding
The project was supported by the Russian Science Foundation (Agreement No. 20-75-10157 dated August 14, 2020 “Studying the possibilities of obtaining recombinant strains of oncolytic viruses with tumor-specific replication and expression of immunomodulatory proteins”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare they have no conflicts of interest. This study does not contain any research involving humans or animals as research objects.
Rights and permissions
About this article
Cite this article
Vorobyev, P.O., Babaeva, F.E., Panova, A.V. et al. Oncolytic Viruses in the Therapy of Lymphoproliferative Diseases. Mol Biol 56, 684–695 (2022). https://doi.org/10.1134/S0026893322050144
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893322050144