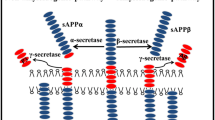
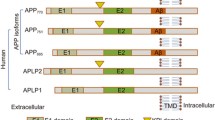
Abstract—
Advances in the research of molecular factors involved in the onset and progression of Alzheimer’s disease, have led to the creation of several pathogenesis concepts of the most common neurodegenerative disease in the world, and amyloid, cholinergic, and neuroinflammatory hypotheses became leading. Over past twenty years, based on these hypotheses, hundreds of drug prototypes were developed, but none of them were able to stop the development of Alzheimer’s disease. In this review, based on the latest experimental data on structure-function properties of chemically modified amyloid-beta isoforms, the concept of the origin and the mechanism of action of amyloid-beta with isomerized Asp7 residue, as a molecular agent of Alzheimer’s disease pathogenesis, is presented. This concept makes it possible not only to combine the most important aspects of existing hypotheses but also indicates ways of creating agents for fighting Alzheimer’s disease with a principally new mechanism of action, “disease-modifying drugs.”
Similar content being viewed by others
REFERENCES
Shelkovnikova T.A., Kulikova A.A., Tsvetkov F.O., Peters O., Bachurin S.O., Bukhman V.L., Ninkina N.N. 2012. Proteinopathies, neurodegenerative disorders with protein aggregation-based pathology. Mol. Biol. (Moscow). 46 (3), 362–374.
Alzheimer'sassociation (2014. 2014 Alzheimer’s disease facts and figures. Alzheimers Dement. 10 (2), e47‒e92.
Rogaev E.I., Sherrington R., Rogaeva E.A., Levesque G., Ikeda M., Liang Y., Chi H., Lin C., Holman K., Tsuda T., Mar L., Sorbi S., Nacmias B., Piacentini S., Amaducci L., et al. 1995. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 376, 775‒778.
Sherrington R., Rogaev E.I., Liang Y., Rogaeva E.A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K., Tsuda T., Mar L., Foncin J.F., Bruni A.C., Montesi M.P., et al. 1995. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 375, 754‒760.
Querfurth H.W., Laferla F.M. 2010. Alzheimer’s disease. N. Engl. J. Med. 362, 329‒344.
Cummings J.L. 2004. Alzheimer’s disease. N. Engl. J. Med.351, 56‒67.
Karran E., Mercken M., De Strooper B. 2011. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 10, 698‒712.
Golde T.E., Dekosky S.T., Galasko D. 2018. Alzheimer’s disease: The right drug, the right time. Science. 362, 1250‒1251.
Müller U.C., Zheng H. 2012. Physiological functions of APP family proteins. Cold Spring Harb. Perspect. Med. 2 (2), a006288.
Roher A.E., Esh C.L., Kokjohn T.A., Castano E.M., Van Vickle G.D., Kalback W.M., Patton R.L., Luehrs D.C., Daugs I.D., Kuo Y.M., Emmerling M.R., Soares H., Quinn J.F., Kaye J., Connor D.J., et al. 2009. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement. 5, 18‒29.
Brothers H.M., Gosztyla M.L., Robinson S.R. 2018. The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer’s disease. Front. Aging Neurosci. 10, 118.
Wang J., Gu B.J., Masters C.L., Wang Y.J. 2017. A systemic view of Alzheimer disease: Insights from amyloid-beta metabolism beyond the brain. Nat. Rev. Neurol. 13, 612‒623.
Cao W., Zheng H. 2018. Peripheral immune system in aging and Alzheimer’s disease. Mol. Neurodegener. 13, 51.
Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. 2018. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. (NY). 4, 575‒590. https://doi.org/10.1016/j.trci.2018.06.014
Morris G., Berk M., Maes M., Puri B.K. 2019. Could Alzheimer’s disease originate in the periphery and if so how so? Mol. Neurobiol. 56, 406‒434.
Esteras N., Alquezar C., De La Encarnacion A., Martin-Requero A. 2016. Lymphocytes in Alzheimer’s disease pathology: Altered signaling pathways. Curr. Alzheimer Res. 13, 439‒449.
Inyushin M.Y., Sanabria P., Rojas L., Kucheryavykh Y., Kucheryavykh L. 2017. Aβ: Peptide originated from platelets promises new strategy in anti-Alzheimer’s drug development. BioMed. Res. Int.2017, 10.
Stewart K.L., Radford S.E. 2017. Amyloid plaques beyond Abeta: A survey of the diverse modulators of amyloid aggregation. Biophys. Rev. 9, 405‒419.
Bush A.I. 2013. The metal theory of Alzheimer’s disease. J. Alzheimer’s Dis. 33, S277‒S281.
Adlard P.A., Parncutt J.M., Finkelstein D.I., Bush A.I. 2010. Cognitive loss in zinc transporter-3 knock-out mice: A phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 30, 1631‒1636.
Friedlich A.L., Lee J.-Y., Van Groen T., Cherny R.A., Volitakis I., Cole T.B., Palmiter R.D., Koh J.-Y., Bush A.I. 2004. Neuronal zinc exchange with the blood vessel wall promotes cerebral amyloid angiopathy in an animal model of Alzheimer’s disease. J. Neurosci. 24, 3453‒3459.
Lee J.Y., Cole T.B., Palmiter R.D., Suh S.W., Koh J.Y. 2002. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc. Natl. Acad. Sci. U. S. A.99, 7705‒7710.
Lovell M.A., Robertson J.D., Teesdale W.J., Campbell J.L., Markesbery W.R. 1998. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 158, 47‒52.
Miller L.M., Wang Q., Telivala T.P., Smith R.J., Lanzirotti A., Miklossy J. 2006. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer’s disease. J. Struct. Biol. 155, 30‒37.
Xie X.M., Smart T.G. 1991. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 349, 521‒524.
Lee M.C., Yu W.C., Shih Y.H., Chen C.Y., Guo Z.H., Huang S.J., Chan J.C.C., Chen Y.R. 2018. Zinc ion rapidly induces toxic, off-pathway amyloid-beta oligomers distinct from amyloid-beta derived diffusible ligands in Alzheimer’s disease. Sci. Rep. 8, 4772.
Hampel H., Mesulam M.M., Cuello A.C., Farlow M.R., Giacobini E., Grossberg G.T., Khachaturian A.S., Vergallo A., Cavedo E., Snyder P.J., Khachaturian Z.S. 2018. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 141, 1917‒1933.
Cummings J., Morstorf T., Zhong K. 2014. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer’s Res. Ther. 6, 37.
Auld D.S., Kornecook T.J., Bastianetto S., Quirion R. 2002. Alzheimer’s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog. Neurobiol. 68, 209‒245.
Perry E.K., Morris C.M., Court J.A., Cheng A., Fairbairn A.F., Mckeith I.G., Irving D., Brown A., Perry R.H. 1995. Alteration in nicotine binding sites in Parkinson’s disease, Lewy body dementia and Alzheimer’s disease: Possible index of early neuropathology. Neuroscience. 64, 385‒395.
Paterson D., Nordberg A. 2000. Neuronal nicotinic receptors in the human brain. Prog. Neurobiol. 61, 75‒111.
Wu J., Kuo Y.-P., George A.A., Xu L., Hu J., and Lukas R.J. 2004. β-Amyloid directly inhibits human α4β2-nicotinic acetylcholine receptors heterologously expressed in human SH-EP1 cells. J. Biol. Chem. 279, 37842‒37851.
Sabri O., Meyer P.M., Graf S., Hesse S., Wilke S., Becker G.A., Rullmann M., Patt M., Luthardt J., Wagenknecht G., Hoepping A., Smits R., Franke A., Sattler B., Tiepolt S., et al. 2018. Cognitive correlates of alpha4beta2 nicotinic acetylcholine receptors in mild Alzheimer’s dementia. Brain. 141, 1840‒1854.
Lawrence J.L.M., Tong M., Alfulaij N., Sherrin T., Contarino M., White M.M., Bellinger F.P., Todorovic C., Nichols R.A. 2014. Regulation of presynaptic Ca2+, synaptic plasticity and contextual fear conditioning by a N-terminal β-amyloid fragment. J. Neurosci. 34, 14210‒14218.
Mediannikov O., Morozov A. 2014). Peptide compound useful for inhibiting amyloid plaque formation. France Patent 2,966,827, filed October 10, 2010; issued August 22, 2014.
Baker H.F., Ridley R.M., Duchen L.W., Crow T.J., Bruton C.J. 1994. Induction of beta (A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Mol. Neurobiol. 8, 25‒39.
Ridley R.M., Baker H.F., Windle C.P., Cummings R.M. 2006. Very long term studies of the seeding of beta-amyloidosis in primates. J. Neural. Transm. 113, 1243‒1251.
Langer F., Eisele Y.S., Fritschi S.K., Staufenbiel M., Walker L.C., Jucker M. 2011. Soluble Abeta seeds are potent inducers of cerebral beta-amyloid deposition. J. Neurosci. 31, 14488‒14495.
Morales R., Duran-Aniotz C., Castilla J., Estrada L.D., Soto C. 2012. De novo induction of amyloid-β deposition in vivo.Mol. Psychiatry. 17, 1347‒1353.
Rosen R.F., Fritz J.J., Dooyema J., Cintron A.F., Hamaguchi T., Lah J.J., Levine H., 3rd, Jucker M., Walker L.C. 2012. Exogenous seeding of cerebral beta-amyloid deposition in betaAPP-transgenic rats. J. Neurochem. 120, 660‒666.
Watts J.C., Giles K., Grillo S.K., Lemus A., Dearmond S.J., Prusiner S.B. 2011. Bioluminescence imaging of Aβ deposition in bigenic mouse models of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A.108, 2528‒2533.
Eisele Y.S., Bolmont T., Heikenwalder M., Langer F., Jacobson L.H., Yan Z.X., Roth K., Aguzzi A., Staufenbiel M., Walker L.C., Jucker M. 2009. Induction of cerebral beta-amyloidosis: Intracerebral versus systemic Abeta inoculation. Proc. Natl. Acad. Sci. U. S. A.106, 12926‒12931.
Eisele Y.S., Obermuller U., Heilbronner G., Baumann F., Kaeser S.A., Wolburg H., Walker L.C., Staufenbiel M., Heikenwalder M., Jucker M. 2010. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 330, 980‒982.
Meyer-Luehmann M., Coomaraswamy J., Bolmont T., Kaeser S., Schaefer C., Kilger E., Neuenschwander A., Abramowski D., Frey P., Jaton A.L., Vigouret J.M., Paganetti P., Walsh D.M., Mathews P.M., Ghiso J., et al. 2006. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 313, 1781‒1784.
Meyer-Luehmann M., Spires-Jones T., Prada C., Garcia-Alloza M., De Calignon A., Rozkalne A., Koenigsknecht-Talboo J., Holtzman D., Bacskai B., Hyman B. 2008. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 451, 720‒724.
Mezias C., Raj A. 2017. Analysis of amyloid-β pathology spread in mouse models suggests spread is driven by spatial proximity, not connectivity. Front. Neurol. 8, 653.
Novotny R., Langer F., Mahler J., Skodras A., Vlachos A., Wegenast-Braun B.M., Kaeser S.A., Neher J.J., Eisele Y.S., Pietrowski M.J., Nilsson K.P., Deller T., Staufenbiel M., Heimrich B., Jucker M. 2016. Conversion of synthetic Abeta to in vivo active seeds and amyloid plaque formation in a hippocampal slice culture model. J. Neurosci. 36, 5084‒5093.
Jucker M., Walker L.C. 2018. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 21, 1341‒1349.
Jaunmuktane Z., Mead S., Ellis M., Wadsworth J.D., Nicoll A.J., Kenny J., Launchbury F., Linehan J., Richard-Loendt A., Walker A.S., Rudge P., Collinge J., Brandner S. 2015. Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature. 525, 247‒250.
Purro S.A., Farrow M.A., Linehan J., Nazari T., Thomas D.X., Chen Z., Mengel D., Saito T., Saido T., Rudge P., Brandner S., Walsh D.M., Collinge J. 2018. Transmission of amyloid-beta protein pathology from cadaveric pituitary growth hormone. Nature. 564, 415‒419.
Masters C.L., Selkoe D.J. 2012. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harbor Persp. Med. 2, a006262.
Portelius E., Price E., Brinkmalm G., Stiteler M., Olsson M., Persson R., Westman-Brinkmalm A., Zetterberg H., Simon A.J., Blennow K. 2011. A novel pathway for amyloid precursor protein processing. Neurobiol. Aging. 32, 1090‒1098.
Kozin S.A., Zirah S., Rebuffat S., Hui Bon Hoa G., Debey P. 2001. Zinc binding to Alzheimer’s Aβ(1‒16) peptide results in stable soluble complex. Biochem. Biophys. Res. Commun. 285, 959‒964.
Zirah S., Kozin S.A., Mazur A.K., Blond A., Cheminant M., Segalas-Milazzo I., Debey P., Rebuffat S. 2006. Structural changes of region 1–16 of the Alzheimer disease amyloid β-peptide upon zinc binding and in vitro aging. J. Biol. Chem. 281, 2151‒2161.
Nisbet R.M., Nuttall S.D., Robert R., Caine J.M., Dolezal O., Hattarki M., Pearce L.A., Davydova N., Masters C.L., Varghese J.N., Streltsov V.A. 2013. Structural studies of the tethered N-terminus of the Alzheimer’s disease amyloid-β peptide. Proteins: Struct. Funct. Bioinformatics. 81, 1748‒1758.
Adzhubei A.A., Anashkina A.A., Makarov A.A. 2017. Left-handed polyproline-II helix revisited: Proteins causing proteopathies. J. Biomol. Struct. Dyn. 35, 2701‒2713.
Adzhubei A.A., Sternberg M.J.E., Makarov A.A. 2013. Polyproline-II helix in proteins: Structure and function. J. Mol. Biol. 425, 2100‒2132.
Tsvetkov P.O., Kulikova A.A., Golovin A.V., Tkachev Y.V., Archakov A.I., Kozin S.A., Makarov A.A. 2010. Minimal Zn2+ binding site of amyloid-β. Biophys. J.99, L84‒L86.
Kozin S.A., Mezentsev Y.V., Kulikova A.A., Indeykina M.I., Golovin A.V., Ivanov A.S., Tsvetkov P.O., Makarov A.A. 2011. Zinc-induced dimerization of the amyloid-β metal-binding domain 1–16 is mediated by residues 11–14. Mol. BioSystems. 7, 1053‒1055.
Kulikova A.A., Tsvetkov P.O., Indeykina M.I., Popov I.A., Zhokhov S.S., Golovin A.V., Polshakov V.I., Kozin S.A., Nudler E., Makarov A.A. 2014. Phosphorylation of Ser8 promotes zinc-induced dimerization of the amyloid-beta metal-binding domain. Mol. Biosyst. 10, 2590‒2596.
Miller Y., Ma B., Nussinov R. 2010. Zinc ions promote Alzheimer Abeta aggregation via population shift of polymorphic states. Proc. Natl. Acad. Sci. U. S. A.107, 9490‒9495.
Istrate A.N., Kozin S.A., Zhokhov S.S., Mantsyzov A.B., Kechko O.I., Pastore A., Makarov A.A., Polshakov V.I. 2016. Interplay of histidine residues of the Alzheimer’s disease Aβ peptide governs its Zn-induced oligomerization. Sci. Rep. 6, 21734.
Kozin S.A., Barykin E.P., Mitkevich V.A., Makarov A.A. 2018. Antiamyloid therapy of Alzheimer’s disease: Current state and prospects. Biochemistry (Moscow). 83 (9), 1057‒1067.
Kulikova A.A., Makarov A.A., Kozin S.A. 2015. Roles of zinc ions and structural polymorphism of β-amyloid in the development of Alzheimer’s disease. Mol. Biol. (Moscow). 49 (2), 217–230.
Mezentsev Y.V., Medvedev A.E., Kechko O.I., Makarov A.A., Ivanov A.S., Mantsyzov A.B., Kozin S.A. 2016. Zinc-induced heterodimer formation between metal-binding domains of intact and naturally modified amyloid-beta species: implication to amyloid seeding in Alzheimer’s disease? J. Biomol. Struct. Dyn. 34, 2317‒2326.
Tsvetkov P.O., Popov I.A., Nikolaev E.N., Archakov A.I., Makarov A.A., Kozin S.A. 2008. Isomerization of the Asp7 residue results in zinc-induced oligomerization of Alzheimer’s disease amyloid β(1‒16) peptide. Chembiochem. 9, 1564‒1567.
Hosoda R., Saido T.C., Otvos L.J., Arai T., Mann D.M.A., Lee V.M.-Y., Trojanowski J.Q., Iwatsubo T. 1998. Quantification of modified amyloid β peptides in Alzheimer disease and Down Syndrome brains. J. Neuropathol. Exp. Neurol. 57, 1089‒1095.
Roher A.E., Lowenson J.D., Clarke S., Wolkow C., Wang R., Cotter R.J., Reardon I.M., Zurcher-Neely H.A., Heinrikson R.L., Ball M.J., Greenberg B.D. 1993. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer’s disease. J. Biol. Chem. 268, 3072‒3083.
Orpiszewski J., Schormann N., Kluve-Beckerman B., Liepnieks J.J., Benson M.D. 2000. Protein aging hypothesis of Alzheimer disease. FASEB J.14, 1255‒1263.
Moro M.L., Phillips A.S., Gaimster K., Paul C., Mudher A., Nicoll J.A.R., Boche D. 2018. Pyroglutamate and isoaspartate modified amyloid-beta in ageing and Alzheimer’s disease. Acta Neuropathol. Commun.6, 3.
Mitkevich V.A., Petrushanko I.Y., Yegorov Y.E., Simonenko O.V., Vishnyakova K.S., Kulikova A.A., Tsvetkov P.O., Makarov A.A., Kozin S.A. 2013. Isomerization of Asp7 leads to increased toxic effect of amyloid-β42 on human neuronal cells. Cell Death Dis. 4, e939.
Zatsepina O.G., Kechko O.I., Mitkevich V.A., Kozin S.A., Yurinskaya M.M., Vinokurov M.G., Serebryakova M.V., Rezvykh A.P., Evgen’ev M.B., Makarov A.A. 2018. Amyloid-β with isomerized Asp7 cytotoxicity is coupled to protein phosphorylation. Sci. Rep. 8, 3518.
Li Y., Nowotny P., Holmans P., Smemo S., Kauwe J.S., Hinrichs A.L., Tacey K., Doil L., Van Luchene R., Garcia V., Rowland C., Schrodi S., Leong D., Gogic G., Chan J., et al. 2004. Association of late-onset Alzheimer’s disease with genetic variation in multiple members of the GAPD gene family. Proc. Natl. Acad. Sci. U. S. A.101, 15688‒15693.
Itakura M., Nakajima H., Kubo T., Semi Y., Kume S., Higashida S., Kaneshige A., Kuwamura M., Harada N., Kita A., Azuma Y.-T., Yamaji R., Inui T., Takeuchi T. 2015. Glyceraldehyde-3-phosphate dehydrogenase aggregates accelerate amyloid-β amyloidogenesis in Alzheimer disease. J. Biol. Chem. 290, 26072‒26087.
Kozin S.A., Cheglakov I.B., Ovsepyan A.A., Telegin G.B., Tsvetkov P.O., Lisitsa A.V., Makarov A.A. 2013. Peripherally applied synthetic peptide isoAsp7-Aβ(1‒42) triggers cerebral β-amyloidosis. Neurotox. Res.24, 370‒376.
Bu X.L., Xiang Y., Jin W.S., Wang J., Shen L.L., Huang Z.L., Zhang K., Liu Y.H., Zeng F., Liu J.H., Sun H.L., Zhuang Z.Q., Chen S.H., Yao X.Q., Giunta B., et al. 2018. Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol. Psychiatry. 23, 1948‒1956.
Burwinkel M., Lutzenberger M., Heppner F.L., Schulz-Schaeffer W., Baier M. 2018. Intravenous injection of beta-amyloid seeds promotes cerebral amyloid angiopathy (CAA). Acta Neuropathol. Commun. 6, 23.
Kulikova A.A., Cheglakov I.B., Kukharsky M.S., Ovchinnikov R.K., Kozin S.A., Makarov A.A. 2016. Intracerebral Injection of metal-binding domain of Abeta comprising the isomerized Asp7 increases the amyloid burden in transgenic mice. Neurotox. Res. 29, 551‒557.
Istrate A.N., Tsvetkov P.O., Mantsyzov A.B., Kulikova A.A., Kozin S.A., Makarov A.A., Polshakov V.I. 2012. NMR solution structure of rat Aβ(1‒16): Toward understanding the mechanism of rats’ resistance to Alzheimer’s disease. Biophys. J.102, 136‒143.
Edrey Y.H., Medina D.X., Gaczynska M., Osmulski P.A., Oddo S., Caccamo A., Buffenstein R. 2013. Amyloid beta and the longest-lived rodent: The naked mole-rat as a model for natural protection from Alzheimer’s disease. Neurobiol. Aging. 34, 2352‒2360.
Ardiles A.O., Tapia-Rojas C.C., Mandal M., Alexandre F., Kirkwood A., Inestrosa N.C., Palacios A.G. 2012. Postsynaptic dysfunction is associated with spatial and object recognition memory loss in a natural model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A.109, 13835‒13840.
Bourdenx M., Dovero S., Thiolat M.-L., Bezard E., Dehay B. 2017. Lack of spontaneous age-related brain pathology in Octodon degus: A reappraisal of the model. Sci. Repts. 7, 45831.
Steffen J., Krohn M., Paarmann K., Schwitlick C., Brüning T., Marreiros R., Müller-Schiffmann A., Korth C., Braun K., Pahnke J. 2016. Revisiting rodent models: Octodon degus as Alzheimer’s disease model? Acta Neuropathol. Commun.4, 91.
Tsvetkov P.O., Cheglakov I.B., Ovsepyan A.A., Mediannikov O.Y., Morozov A.O., Telegin G.B., Kozin S.A. 2015. Peripherally applied synthetic tetrapeptides HAEE and RADD slow down the development of cerebral beta-amyloidosis in AbetaPP/PS1 transgenic mice. J. Alzheimers Dis. 46, 849‒853.
Barykin E.P., Petrushanko I.Y., Kozin S.A., Telegin G.B., Chernov A.S., Lopina O.D., Radko S.P., Mitkevich V.A., Makarov A.A. 2018. Phosphorylation of the amyloid-beta peptide inhibits zinc-dependent aggregation, prevents Na,K-ATPase inhibition, and reduces cerebral plaque deposition. Front. Mol. Neurosci. 11, 302.
Kozin S.A., Barykin E.P., Telegin G.B., Chernov A.S., Adzhubei A.A., Radko S.P., Mitkevich V.A., Makarov A.A. 2018. Intravenously injected amyloid-β peptide with isomerized Asp7 and phosphorylated Ser8 residues inhibits cerebral β-amyloidosis in AβPP/PS1 transgenic mice model of Alzheimer’s disease. Front. Neurosci.12, 518.
Funding
The work is supported by Russian Science Foundation (grant no. 19-74-30007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Khaitin
Rights and permissions
About this article
Cite this article
Kozin, S.A., Makarov, A.A. The Convergence of Alzheimer’s Disease Pathogenesis Concepts. Mol Biol 53, 896–903 (2019). https://doi.org/10.1134/S0026893319060104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893319060104