Abstract—
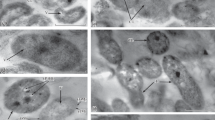
Azospirillum brasilense forms biofilms on various surfaces. It was previously shown that inactivation of the ahpC gene of alkyl hydroperoxide reductase in the SK586 mutant of A. brasilense Sp245 led to an increased sensitivity to peroxides and to changes in flagellation, motility, and cell surface properties. This work revealed that under nitrogen limitation, Sp245 switches predominantly to the biofilm mode of life, whereas this tendency is not characteristic of the mutant SK586. Inactivation of the ahpC gene negatively affects the viability of SK586 in biofilms, which is most noticeable under nitrogen limitation. While no significant morphological differences were found between cyst-like forms of Sp245 and SK586, the dormant mutant forms grown in a nitrogen-free medium were more sensitive to hydrogen peroxide than the dormant forms of Sp245. In Sp245 biofilms, viable forms resistant to drying were found even after 9 months of storage, while in SK586 biofilms, such bacteria remained viable for less than a month. In the course of adaptation to the plant root system, the number of Azospirillum cells resistant to drying increased, as compared to the number of such cells from mature biofilms on abiotic surfaces. Unlike Sp245, SK586 did not promote the development of the root system and the growth of the aerial parts of 10-day-old plants, possibly owing to its lower ability to colonize the roots of wheat seedlings incubated in a liquid medium. Alkyl hydroperoxide reductase is probably essential for better resistance of azospirilla to various stresses and for manifestation of their plant-growth-promoting activity.



Similar content being viewed by others
REFERENCES
Baldani, V.L.D., Baldani, J.I., and Döbereiner, J., Effects of Azospirillum inoculation on root infection and nitrogen incorporation in wheat, Can. J. Microbiol., 1983, vol. 29, no. 8, pp. 924–929.
Bashan, Y. and de-Bashan, L.E., How the plant growth-promoting bacterium Azospirillum promotes plant growth – a critical assessment, Adv. Agron., 2010, vol. 108, pp. 77–136.
Berg, R.H., Tyler, M.E., Novick, N.J., Vasil, V., and Vasil, I.K., Biology of Azospirillum–sugarcane association: enhancement of nitrogenase activity, Appl. Environ. Microbiol., 1980, vol. 39, no. 3, pp. 642–649.
Bogino, P.C., Oliva, M.M., Sorroche, F.G., and Giordano, W., The role of bacterial biofilms and surface components in plant-bacterial associations, Int. J. Mol. Sci., 2013, vol. 14, pp. 15838–15859.
Clara, R.W. and Knowles, R., Superoxide dismutase, catalase, and peroxidase in ammonium-grown and nitrogen-fixing Azospirillum brasilense, Can. J. Microbiol., 1984, vol. 30, pp. 1222–1228.
Döbereiner, J. and Day, J.M., Associative symbiosis in tropical grass: characterization of microorganisms and dinitrogen fixing sites, in Symposium on Nitrogen Fixation, Newton, W.E. and Nijmans, C.J., Eds., Pullman: Washington State Univ., 1976, pp. 518–538.
Elmas, M., Alexiades, V., O’Neal, L., and Alexandre, G., Modeling aerotaxis band formation in Azospirillum brasilense, BMC Microbiol., 2019, vol. 19, p. 101.
Fibach-Paldi, S., Burdman, S., and Okon, Y., Key physiological properties contributing to rhizosphere adaptation and plant growth promoting abilities of Azospirillum brasilense, FEMS Microbiol. Lett., 2012, vol. 326, no. 2, pp. 99–108.
Flemming, H.-C. and Wingender, J., The biofilm matrix, Nat. Rev. Microbiol., 2010, vol. 8, no. 9, pp. 623–633.
Fukami, J., Cerezini, P., and Hungria, M., Azospirillum: benefits that go far beyond biological nitrogen fixation, AMB Expr., 2018, vol. 8, pp. 73–85.
Gómez-Villalba, B., Calvo, C., Vilchez, R., González-López, J., and Rodelas, B., TGGE analysis of the diversity of ammonia-oxidizing and denitrifying bacteria in submerged filter biofilms for the treatment of urban wastewater, Appl. Microbiol. Biotechnol., 2006, vol. 72, no. 2, pp. 393–400.
Kamariah, N., Eisenhaber, B., Eisenhaber, F., and Grüber, G., Molecular mechanism of the Escherichia coli AhpC in the function of a chaperone under heat-shock conditions, Sci. Rep., 2018, vol. 8, p. 14151.
Lee, J.T., Lee, S.S., Mondal, S., Tripathi, B.N., Kim, S., Lee, K.W., Hong, S.H., Bai, H.-W., Cho, J.-Y., and Chung, B.Y., Enhancement of the chaperone activity of alkyl hydroperoxide reductase C from Pseudomonas aeruginosa PAO1 resulting from a point-specific mutation confers heat tolerance in Escherichia coli, Mol. Cells, 2016, vol. 39, no. 8, pp. 594–602.
Nozhevnikova, A.N., Botchkova, E.A., and Plakunov, V.K., Multi-species biofilms in ecology, medicine, and biotechnology, Microbiology (Moscow), 2015, vol. 84, no. 6, pp. 731–750.
Nur, I., Okon, Y., and Henis, Y., Effect of dissolved oxygen tension on production of carotenoids, poly-β-hydroxybutyrate, succinate oxidase and superoxide dismutase by Azospirillum brasilense grown in continuous culture, J. Gen. Microbiol., 1982, vol. 128, pp. 2937–2943.
O’Toole, G.A. and Kolter, R., Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis, Mol. Microbiol., 1998, vol. 28, no. 3, pp. 449–461.
Plakunov, V.K., Mart’yanov, S.V., Teteneva, N.A., and Zhurina, M.V., A universal method for quantitative characterization of growth and metabolic activity of microbial biofilms in static models, Microbiology (Moscow), 2016, vol. 85, no. 4, pp. 509–513.
Poole, L.B. and Ellis, H.R., Flavin-dependent alkyl hydroxyperoxide reductase from Salmonella typhymurium. 1. Purification and enzymatic activities of over expressed AhpF and AhpC proteins, Biochemistry, 1996, vol. 35, pp. 56–64.
Ramírez-Mata, A., López-Lara, L.I., Xiqui-Vázquez, L., Jijón-Moreno, S., Romero-Osorio, A., and Baca, B.E., The cyclic-di-GMP diguanylate cyclase CdgA has a role in biofilm formation and exopolysaccharide production in Azospirillum brasilense, Res. Microbiol., 2016, vol. 167, pp. 190–201.
Sambrook, J., Fritsch, E.F., and Maniatis, T., Molecular Cloning: A Laboratory Manual, 2nd ed., New York: Cold Spring Harbor Laboratory Press, 1989.
Scheludko, A.V., Katsy, E.I., Ostudin, N.A., Gringaus, O.K., and Panasenko, V.I., Novel classes of Azospirillum brasilense mutants with defects in the assembly and functioning of polar and lateral flagella, Mol. Genet. Mikrobiol. Virusol., 1998, no. 4, pp. 33–37.
Schloter, M. and Hartmann, A., Endophytic and surface colonization of wheat roots (Triticum aestivum) by different Azospirillum brasilense strains studied with strain-specific monoclonal antibodies, Symbiosis, 1998, vol. 25, pp. 159–179.
Shelud’ko, A.V., Filip’echeva, Y.A., Shumilova, E.M., Kh-lebtsov, B.N., Burov, A.M., Petrova, L.P., and Katsy, E.I., Changes in biofilm formation in the nonflagellated flhB1 mutant of Azospirillum brasilense Sp245, Microbiology (Moscow), 2015, vol. 84, no. 2, pp. 144–151.
Shelud’ko, A.V., Filip’echeva, Y.A., Telesheva, E.M., Burov, A.M., Evstigneeva, S.S., Burygin, G.L., and Petrova, L.P., Characterization of carbohydrate-containing components of Azospirillum brasilense Sp245 biofilms, Microbiology (Moscow), 2018, vol. 87, no. 5, pp. 610–620.
Shelud’ko, A.V., Mokeev, D.I., Evstigneeva, S.S., Fi-lip’echeva, Yu.A., Burov, A.M., Petrova, L.P., Pono-mareva, E.G., and Katsy, E.I., Cell ultrastructure in biofilms of Azospirillum brasilense, Microbiology (Moscow), 2020, vol. 89, no. 1, pp. 50–63.
Shelud’ko, A.V., Shirokov, A.A., Sokolova, M.K., Sokolov, O.I., Petrova, L.P., Matora, L.Yu., and Katsy, E.I., Wheat root colonization by Azospirillum brasilense strains with different motility, Microbiology (Moscow), 2010, vol. 79, no. 5, pp. 688–695.
Tarrand, J.J, Krieg, N.R., and Döbereiner, J., A taxonomic study of the Spirillum lipoferum group with description of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum braslense sp. nov., Can. J. Microbiol., 1978, vol. 24, no. 8, pp. 967–980.
Vasil’eva, G.G., Glyan’ko, A.G., Mironova, N.V., Putilina, T.E., and Luzova G.B., Active oxygen species in pea seedlings during the interactions with symbiotic and pathogenic microorganisms, Appl. Biochem. Microbiol., 2007, vol. 43, no. 2, pp. 217–221.
Wang, D., Xu, A., Elmerich, C., and Ma, L.Z., Biofilm formation enables free-living nitrogen-fixing rhizobacteria to fix nitrogen under aerobic conditions, ISME J., 2017, vol. 11, pp. 1602–1613.
Wasim, M., Bible, A.N., Xie, Z., and Alexandre, G., Alkyl hydroperoxide reductase has a role in oxidative stress resistance and in modulating changes in cell-surface properties in Azospirillum brasilense Sp245, Microbiology (SGM), 2009, vol. 155, pp. 1192–1202.
ACKNOWLEDGMENTS
The authors wish to thank the Collection of Rhizosphere Microorganisms of the Institute of Biochemistry and Physiology of Plants and Microorganisms, Russian Academy of Sciences (IBPPM RAS), for A. brasilense strain Sp245 [IBPPM 219] and the IBPPM Simbioz Center for the Collective Use of Research Equipment in the Field of Physical–Chemical Biology and Nanobiotechnology (Saratov, Russia) for the use of the Multiskan Ascent, Leica DM6000 B, and Libra 120 devices.
Funding
This study was funded in part by the RFBR, project number 20-04-00006-a.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies involving animals performed by any of the authors.
The authors declare that they have no conflict of interests.
Additional information
Translated by A. Oleskin
Rights and permissions
About this article
Cite this article
Shelud’ko, A.V., Mokeev, D.I., Evstigneeva, S.S. et al. Suppressed Biofilm Formation Efficiency and Decreased Biofilm Resistance to Oxidative Stress and Drying in an Azospirillum brasilense ahpC Mutant. Microbiology 90, 56–65 (2021). https://doi.org/10.1134/S0026261721010100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261721010100