Abstract
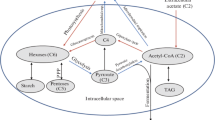
Purple bacteria are able to use H2 for photoautotrophic, photomixotrophic, and chemoautotrophic growth, exhibiting high metabolic lability. Depending on the type of metabolism, hydrogen may be consumed with release of energy and/or reductive equivalents. Purple bacteria may also release H2 as a terminal electron acceptor or in the course of dinitrogen fixation. Thus, hydrogen metabolism in purple bacteria is diverse; these bacteria are often used as models for investigation of the metabolic traits and interrelation of the metabolic pathways involving molecular hydrogen. In this review, the present-day state of investigation of hydrogen metabolism in purple bacteria is reflected and its possible practical applications are discussed. Nitrogenase and hydrogenase, the major key enzymes of hydrogen metabolism, are discussed in brief. A generalized scheme of H2 role in the metabolism of purple bacteria is presented. Experimental approaches for investigation of the rates of hydrogen production are discussed. Immobilized systems are noted as the most promising approach for development of model systems for hydrogen production.
Similar content being viewed by others
References
Lyon, E.J., Shima, S., Buurman, G., Chowdhuri, S., Batschauer, A., Steinbach, K., and Thauer, R.K., UV-A/blue-light inactivation of the ‘metal-free’ hydrogenase (Hmd) from methanogenic archaea—the enzyme contains functional iron after all, Eur. J. Biochem., 2004, vol. 271, pp. 195–204.
Shima, S. and Thauer, R.K., A third type of hydrogenase catalyzing H2 activation, Chem. Record., 2007, vol. 7, pp. 37–46.
Vignais, P. and Billoud, B., Occurrence, classification, and biological function of hydrogenases: an overview, Chem. Rev., 2007, vol. 107, pp. 4206–4272.
Laurinavichene, T.V., Zorin, N.A., and Tsygankov, A.A., Effect of redox potential on activity of hydrogenase 1 and hydrogenase 2 in Escherichia coli, Arch. Microbiol., 2002, vol. 178, pp. 437–442.
Serebryakova, L.T. and Sheremetieva, M.E., Characterization of catalytic properties of hydrogenase isolated from the unicellular cyanobacterium Gloeocapsa alpicola Calu 743, Biochemistry (Moscow), 2006), vol. 71, pp. 1370–1376.
Meyer, J., [FeFe] hydrogenases and their evolution: a genomic perspective, Cell. Mol. Life Sci., 2007, vol. 64, pp. 1063–1084.
Abo-Hashesha, M., Sabourin-Provost, G., and Hallenbeck, P.C., HydA is inactive when overexpressed in Rhodospirillum rubrum but can be matured in Escherichia coli, Int. J. Hydr. Energy, vol. 38, pp. 11233–11240.
Kim, E.J., Lee, M.K., Kim, M.S., and Lee, J.K., Molecular hydrogen production by nitrogenase of Rhodobacter sphaeroides and by Fe-only hydrogenase of Rhodospirillum rubrum, Int. J. Hydrogen Energy, 2008, vol. 33, pp. 1516–1521.
Korbas, M., Vogt, S., Meyer-Klaucke, W., Bill, E., Lyon, E.J., Thauer, R.K., and Shima, S., The ironsulfur cluster-free hydrogenase (Hmd) is a metalloenzyme with a novel iron binding motif, J. Biol. Chem., 2006, vol. 281, pp. 30804–30813.
Kroger, A., Biel, S., Simon, J., Gross, R., Unden, G., and Lancaster, C.R.D., Fumarate respiration of Wolinella succinogenes: enzymology, energetics and coupling mechanism, Biochim. Biophys. Acta, 2002, vol. 1553, nos. 1–2, pp. 23–38.
Wu, L.F., Chanal, A., and Rodrigue, A., Membrane targeting and translocation of bacterial hydrogenases, Arch. Microbiol., 2000, vol. 173, no. 5, pp. 319–324.
Leclerc, M., Colbeau, A., Cauvin, B., and Vignais, P.M., Cloning and sequencing of the genes encoding the large and the small subunits of the H2 uptake hydrogenase (Hup) of Rhodobacter capsulatus, Mol. Gen. Genet., 1988, vol. 214, pp. 97–107.
Cauvin, B., Colbeau, A., and Vignais, P.M., The hydrogenase structural operon in Rhodobacter capsulatus contains a third gene, hupM, necessary for the formation of a physiologically competent hydrogenase, Mol. Microbiol., 1991, vol. 5, pp. 2519–2527.
Vignais, P.M., Billoud, B., and Meyer, J., Classification and phylogeny of hydrogenases, FEMS Microbiol. Rev., 2001, vol. 25, pp. 455–501.
Maness, P.C. and Weaver, P.F., Evidence for three distinct hydrogenase activities in Rhodospirillum rubrum, Appl. Microbiol. Biotechnol., 2001, vol. 57, pp. 751–756.
Colbeau, A., Kovacs, K.L., Chabert, J., and Vignais, P.M., Cloning and sequences of the structural (hupSLC) and accessory (hupDHI) genes for hydrogenase biosynthesis in Thiocapsa roseopersicina, Gene, 1994, vol. 140, pp. 25–31.
Uffen, R.L., Colbeau, A., Richaud, P., and Vignais, P.M., Cloning and sequencing the genes encoding uptake-hydrogenase subunits of Rhodocyclus gelatinosus, Mol. Gen. Genet., 1990, vol. 221, pp. 49–58.
Serebryakova, L.T., Zorin, N.A., and Gogotov, I.N., Isolation and properties of hydrogenase from Rhodopseudomonas capsulata, Biochemistry, 1984, vol. 49, no. 9, pp. 1241–1247.
Rakhely, G., Colbeau, A., Garin, J., Vignais, P.M., and Kovacs, K.L., Unusual organization of the genes coding for HydSL, the stable [NiFe] hydrogenase in the photosynthetic bacterium Thiocapsa roseopersicina BBS, J. Bacteriol., 1998, vol. 180, pp. 1460–1465.
Long, M.N., Liu, J.J., Chen, Z.F., Bleijlevens, B., Roseboom, W., and Albracht, S.P.J., Characterization of a hoxefuyh type of [NiFe] hydrogenase from Allochromatium vinosum and some EPR and IR properties of the hydrogenase module, J. Biol. Inorgan. Chem., 2007, vol. 12, pp. 62–78.
Kovacs, K.L., Fodor, B., Kovacs, A.T., Csanadi, G., Maroti, G., Balogh, J., Arvani, S., and Rakhely, G., Hydrogenases, accessory genes and the regulation of NiFe hydrogenase biosynthesis in Thiocapsa roseopersicina, Int. J. Hydrogen Energy, 2002, vol. 27, pp. 1463–1469.
Maroti, G., Fodor, B.D., Rakhely, G., Kovacs, A.T., Arvani, S., and Kovacs, K.L., Accessory proteins functioning selectively and pleiotropically in the biosynthesis of NiFe hydrogenases in Thiocapsa roseopersicina, Eur. J. Biochem., 2003, vol. 270, pp. 2218–2227.
Dahl, C., Rakhely, G., Pottsperling, A.S., Fodor, B., Takacs, M., Toth, A., Kraeling, M., Gyorfi, K., Kovacs, A., Tusz, J., and Kovacs, K.L., Genes involved in hydrogen and sulfur metabolism in phototrophic sulfur bacteria, FEMS Microbiol. Lett., 1999, vol. 180, pp. 317–324.
Laurinavichene, T.V., Rakhely, G., Kovacs, K.L., and Tsygankov, A.A., The effect of sulfur compounds on H2 evolution/consumption reactions, mediated by various hydrogenases, in the purple sulfur bacterium, Thiocapsa roseopersicina, Arch. Microbiol., 2007, vol. 188, pp. 403–410.
Gogotov, I.N., Zorin, N.A., Serebriakova, L.T., and Kondratieva, E.N., The properties of hydrogenase from Thiocapsa roseopersicina, Biochim. Biophys. Acta, 1978, vol. 523, pp. 335–343.
Szilagyi, A., Kovacs, K.L., Rakhely, G., and Zavodszky, P., Homology modeling reveals the structural background of the striking difference in thermal stability between two related NiFe hydrogenases, J. Mol. Modeling, 2002, vol. 8, pp. 58–64.
Pandelia, M.E., Lubitz, W., and Nitschke, W., Evolution and diversification of group 1 [NiFe] hydrogenases. Is there a phylogenetic marker for O2-tolerance?, Biochim. Biophys. Acta, 2012, vol. 1817, no. 9, pp. 1565–1575.
Fritsch, J., Scheerer, P., Frielingsdorf, S., Kroschinsky, S., Friedrich, B., Lenz, O., and Spahn, C.M.T., The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulphur center, Nature, 2011, vol. 479, pp. 249–252.
Vincent, K., Cracknell, J., Lenz, O., Zebger, I., Friedrich, B., and Armstrong, F., Electrocatalytic hydrogen oxidation by an enzyme at high carbon monoxide or oxygen levels, Proc. Natl. Acad. Sci. U. S. A., 2005, vol. 102, no. 47, pp. 16951–16954.
Goris, T., Wait, A.F., and Saggu, M., A unique ironsulfur cluster is crucial for oxygen tolerance of a [NiFe]-hydrogenase, Nature Chem. Biol., 2011, vol. 7, no. 5, pp. 648–648.
Vignais, P.M., Dimon, B., Zorin, N.A., Colbeau, A., and Elsen, S., HupUV proteins of Rhodobacter capsulatus can bind H2: evidence from the H-D exchange reaction, J. Bacteriol., 1997, vol. 179, pp. 290–292.
Kovacs, A.T., Rakhely, G., Balogh, J., Maroti, G., Cournac, L., Carrier, P., Meszaros, L.S., Peltier, G., and Kovacs, K.L., Hydrogen independent expression of HupSL genes in Thiocapsa roseopersicina BBS, FEBS J., 2005, vol. 272, pp. 4807–4816.
Fritsch, J., Lenz, O., and Friedrich, B., Structure, function and biosynthesis of O2-tolerant hydrogenases, Nature Rev. Microbiol., 2013, vol. 11, pp. 106–114.
Duche, O., Elsen, S., Cournac, L., and Colbeau, A., Enlarging the gas access channel to the active site renders the regulatory hydrogenase HupUV of Rhodobacter capsulatus O2 sensitive without affecting its transductory activity, FEBS J., 2005, vol. 272, pp. 3899–3908.
Rakhely, G., Kovacs, A.T., Maroti, G., Fodor, B.D., Csanadi, G., Latinovics, D., and Kovacs, K.L., Cyanobacterial-type, heteropentameric, NAD(+)-reducing NiFe hydrogenase in the purple sulfur photosynthetic bacterium Thiocapsa roseopersicina, Appl. Environ. Microbiol., 2004, vol. 70, pp. 722–728.
Appel, J. and Schulz, R., Hydrogen metabolism in organisms with oxygenic photosynthesis: hydrogenases as important regulatory devices for a proper redox poising?, J. Photochem. Photobiol., 1998, vol. 47, pp. 1–11.
Rakhely, G., Laurinavichene, T.V., Tsygankov, A.A., and Kovacs, K.L., The role of Hox hydrogenase in the H2 metabolism of Thiocapsa roseopersicina, BBA-Bioenergetics, 2007, vol. 1767, pp. 671–676.
Ma, K., Schicho, R.N., Kelly, R.M., and Adams, M.W., Hydrogenase of the hyperthermophile Pyrococcus furiosus is an elemental sulfur reductase or sulfhydrogenase: Evidence for a sulfur-reducing hydrogenase ancestor, Proc. Natl. Acad. Sci. U. S. A., 1993, vol. 90, no. 11, pp. 5341–5344.
Singer, S.W., Hirst, M.B., and Ludden, P.W., Codependent H2 evolution by Rhodospirillum rubrum: role of CODH: CooF complex, BBA-Bioenergetics, 2006, vol. 1757, pp. 1582–1591.
Sipma, J., Henstra, A.M., Parshina, S.N., Lens, P.N.L., Lettinga, G., and Stams, A.J.M., Microbial CO conversions with applications in synthesis gas purification and bio-desulfurization, Crit. Rev. Biotechnol., 2006, vol. 26, pp. 41–65.
Oh, Y.K., Kim, Y.J., Park, J.Y., Lee, T.H., Kim, M.S., and Park, S., Biohydrogen production from carbon monoxide and water by Rhodopseudomonas palustris P4, Biotechnol. Bioproc. Eng., 2005, vol. 10, pp. 270–274.
Maeda, T., Sanchez-Torres, V., and Wood, T.K., Metabolic engineering to enhance bacterial hydrogen production, Microb. Biotechnol., 2008, vol. 1, no. 1, pp. 30–39.
Constant, P., Poissant, L., and Villemur, R., Isolation of Streptomyces sp. PCB7, the first microorganism demonstrating high-affinity uptake of tropospheric H2, ISME J., 2008, vol. 2, no. 10, pp. 1066–1076.
Constant, P., Chowdhury, S.P., Hesse, L., Pratscher, J., and Conrad, R., Genome data mining and soil survey for the novel group 5 [NiFe]-hydrogenase to explore the diversity and ecological importance of presumptive high affinity H2-oxidizing bacteria, Appl. Environ. Microbiol., 2011, vol. 77, no. 17, pp. 6027–6035.
Bock, A., King, P.W., Blokesch, M., and Posewitz, M.C., Maturation of hydrogenases, Adv. Microb. Physiol., 2006, vol. 51, pp. 1–71.
Leach, M.R. and Zamble, D.B., Metallocenter assembly of the hydrogenase enzymes, Curr. Opin. Chem. Biol., 2007, vol. 11, pp. 159–165.
Wu, L.F., Berthelot, M.A., Waugh, R., Edmonds, C.J., Holt, S.E., and Boxer, D.H., Nickel deficiency gives rise to the defective hydrogenase phenotype of hydC and fnr mutants in Escherichia coli, Mol. Microbiol., 1989, vol. 3, pp. 1709–1718.
Olson, J.W., Fu, C.L., and Maier, R.J., The HypB protein from Bradyrhizobium japonicum can store nickel and is required for the nickel-dependent transcriptional regulation of hydrogenase, Mol. Microbiol., 1997, vol. 24, pp. 119–128.
Fontecave, M., Choudens, S.O., Py, B., and Barras, F., Mechanisms of iron-sulfur cluster assembly: the SUF machinery, J. Biol. Inorg. Chem., 2005, vol. 10, pp. 713–721.
Schubert, T., Lenz, O., Krause, E., Volkmer, R., and Friedrich, B., Chaperones specific for the membranebound [NiFe]-hydrogenase interact with the Tat signal peptide of the small subunit precursor in Ralstonia eutropha H16, Mol. Microbiol., 2007, vol. 66, pp. 453–467.
Fritsch, J., Lenz, O., and Friedrich, B., Structure, function and biosynthesis of O2-tolerant hydrogenases, Nature Rev. Microbiol., 2013, vol. 11, pp. 106–114.
Parkin, A. and Sargent, F., The hows and whys of aerobic H2 metabolism, Curr. Opin. Chem. Biol., 2012, vol. 16, pp. 26–34.
Friedrich, B., Vignais, P., Lenz, O., and Colbeau, A., Regulation of hydrogenase gene expression, in Hydrogen as a Fuel: Learning from Nature, Cammack, R., Frey, M., and Robson, R., Eds., London: Taylor&Francis, 2001, pp. 33–56.
Hoch, J.A. and Varughese, K.I., Keeping signals straight in phosphorelay signal transduction, J. Bacteriol., 2001, vol. 183, pp. 4941–4949.
Vignais, P.M., Elsen, S., and Colbeau, A., Transcriptional regulation of the uptake [NiFe] hydrogenase genes in Rhodobacter capsulatus, Biochem. Soc. Trans., 2005, vol. 33, pp. 28–32.
Rey, F.E., Oda, Y., and Harwood, C.S., Regulation of uptake hydrogenase and effects of hydrogen utilization on gene expression in Rhodopseudomonas palustris, J. Bacteriol., 2006, vol. 188, pp. 6143–6152.
Laurinavichene, T.V., Vasileva, L.G., Tsygankov, A.A., and Gogotov, I.N., The purple nonsulfur bacterium Rhodobacter sphaeroides, isolated from the rice field soil of South Vietnam, Microbiology (Moscow), 1988, vol. 57, pp. 810–815.
Tsygankov, A.A. and Gogotov, I.N., Influence of temperature and pH of the medium on the nitrogenase and hydrogenase activity of Rhodopseudomonas capsulata in nitrogen fixation, Microbiology (Moscow), 1982, vol. 51, no. 3, pp. 325–330.
Tsygankov, A.A., Gogotov, I.N., and Kondratieva, E.N., Growth of Rhodopseudomonas capsulata and its synthesis of hydrogenase under conditions of continuous culturing, Microbiology (Moscow), 1984, vol. 53, pp. 306–312.
Elsen, S., Colbeau, A., Chabert, J., and Vignais, P.M., The hupTUV operon is involved in negative control of hydrogenase synthesis in Rhodobacter capsulatus, J. Bacteriol., 1996, vol. 178, pp. 5174–5181.
Tsygankov, A.A., Yakunin, A.F., and Gogotov, I.N., Hydrogenase activity of Rhodopseudomonas capsulata growing in organic media, Mikrobiologiya, 1982, vol. 51, pp. 533–537.
Iuchi, S. and Lin, E.C., Adaptation of Escherichia coli to redox environments by gene expression, Mol. Microbiol., 1993, vol. 9, pp. 9–15.
Khoroshilova, N., Popescu, C., Munck, E., Beinert, H., and Kiley, P.J., Iron-sulfur cluster disassembly in the FNR protein of Escherichia coli by O2: [4Fe-4S] to [2Fe-2S] conversion with loss of biological activity, Proc. Natl. Acad. Sci. U. S. A., 1997, vol. 94, pp. 6087–6092.
Kondrat’eva, E.N. and Gogotov, I.N., Molekulyarnyi vodorod v metabolizme mikroorganizmov (Molecular Hydrogen in Microbial Metabolism), Moscow: Nauka, 1981.
Kovacs, A.T., Rakhely, G., Browning, D.F., Fulop, A., Maroti, G., Busby, S.J.W., and Kovacs, K.L., An FNR-type regulator controls the anaerobic expression of hyn hydrogenase in Thiocapsa roseopersicina, J. Bacteriol., 2005, vol. 187, pp. 2618–2627.
Roberts, G.P., Youn, H., and Kerby, R.L., CO-sensing mechanisms, Micrbiol. Mol. Biol. Rev., 2004, vol. 68, pp. 453–473.
Shelver, D., Kerby, R.L., He, Y.P., and Roberts, G.P., CooA, a CO-sensing transcription factor from Rhodospirillum rubrum, is a CO-binding heme protein, Proc. Natl. Acad. Sci. U. S. A., 1997, vol. 94, pp. 11216–11220.
Joshi, H.M. and Tabita, F.R., A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation, Proc. Natl. Acad. Sci. U. S. A., 1996, vol. 93, pp. 14515–14520.
Elsen, S., Dischert, W., Colbeau, A., and Bauer, C.E., Expression of uptake hydrogenase and molybdenum nitrogenase in Rhodobacter capsulatus is coregulated by the RegB-RegA two-component regulatory system, J. Bacteriol., 2000, vol. 182, pp. 2831–2837.
Du, S., Bird, T., and Bauer, C.E., DNA binding characteristics of RegA: a constitutively active anaerobic activator of photosynthesis gene expression in Rhodobacter capsulatus, J. Biol. Chem., 1998, vol. 273, pp. 18509–18513.
Bird, T., Du, S., and Bauer, C.E., Autophosphorylation, phosphotransfer, and DNA-binding properties of the RegB/RegA two-component regulatory system in Rhodobacter capsulatus, J. Biol. Chem., 1999, vol. 274, pp. 16343–16348.
Eady, R.R., Current status of structure function relationships of vanadium nitrogenase, Coord. Chem. Rev., 2003, vol. 237, pp. 23–30.
Hoffman, B.M., Dean, D.R., and Seefeldt, L.C., Climbing nitrogenase: toward a mechanism of enzymatic nitrogen fixation, Acc. Chem. Res., 2009, vol. 42, pp. 609–619.
Seefeldt, L.C., Hoffman, B.M., and Dean, D.R., Mechanism of Mo-dependent nitrogenase, Ann. Rev. Biochem., 2009, vol. 78, pp. 701–722.
Tsygankov, A.A. and Laurinavichene, T.V., Growth and nitrogen fixation by Rhodobacter capsulatus in the presence and absence of Mo, Mikrobiologiya, 1993, vol. 62, pp. 855–862.
Siefert, E. and Pfennig, N., Hydrogen metabolism and nitrogen fixation in wild type and Nif-mutants of Rhodopseudomonas acidophila, Biochimie, 1978, vol. 60, pp. 261–265.
Oda, K., Samanta, S.K., Rey, F.E., Wu, L., Yan, T., Zhou, J., and Harwood, C.S., Functional genomic analysis of three nitrogenase isozymes in the photosynthetic bacterium Rhodopseudomonas palustris, J. Bacteriol., 2005, vol. 187, pp. 7784–7794.
Robson, R.L., Woodley, P.R., Pau, R.N., and Eady, R.R., Structural genes for the vanadium nitrogenase from Azotobacter chroococcum, EMBO J., 1989, vol. 8, pp. 1217–1224.
Schneider, K., Muller, A., Schramm, U., and Klipp, W., Demonstration of a molybdenum-independent and vanadium-independent nitrogenase in a nifhDKdeletion mutant of Rhodobacter capsulatus, Eur. J. Biochem., 1991, vol. 195, pp. 653–661.
Yakunin, A.F., Tsygankov, A.A., Troshina, O.Yu., and Gogotov, I.N., Growth and nitrogenase activity of Rhodobacter capsulatus and Rhodobacter sphaeroides in continuous cultures depending on the presence of Mo, W, and V, in the medium, Mikrobiologiya, 1991, vol. 60, pp. 41–46.
Lehman, L.J. and Roberts, G.P., Identification of an alternative nitrogenase system in Rhodospirillum rubrum, J. Bacteriol., 1991, vol. 173, pp. 5705–5711.
Tsygankov, A.A. and Laurinavichene, T.V., Growth and nitrogenase activity in Rhodobacter capsulatus depending on pH and illumination in the presence and absence of Mo, Mikrobiologiya, 1996, vol. 65, pp. 499–504.
Hinnemann, B. and Norskov, J.K., Catalysis by enzymes: the biological ammonia synthesis, Top. Catal., 2006, vol. 37, pp. 55–70.
Zinchenko, V.V., Genetic regulation of nitrogen assimilation in photosynthetic bacteria, Russ. J. Genet. 1999 vol. 35, no. 11, pp. 1287–1301.
Shestakov, S.V. and Mikheeva, L.E., Genetic control of hydrogen metabolism in cyanobacteria, Russ. J. Genet., 2006, vol. 42, no. 11, pp. 1272–1284.
Zurrer, H. and Bachofen, R., Aspects of growth and hydrogen production of the photosynthetic bacterium Rhodospirillum rubrum in continuous culture, Biomass, 1982, vol. 2, pp. 165–174.
Tsygankov, A.A., Fedorov, A.S., Laurinavichene, T.V., Gogotov, I.N., Rao, K.K., and Hall, D.O., Actual and potential rates of hydrogen photoproduction by continuous culture of the purple non-sulphur bacterium Rhodobacter capsulatus, Appl. Microbiol. Biotechnol., 1998, vol. 49, pp. 102–107.
Basak, N., Jana, A.K., Das, D., and Saikia, D., Photofermentative molecular hydrogen production by purple non sulfur (PNS) bacteria in various modes: The present progress an future perspective, Int. J. Hydrogen Energy, 2014. http://dx.doi.org/10.1016/j.ijhydene.2014.02.093
Tsygankov, A., Hydrogen production by purple bacteria: immobilized vs suspension cultures, in Biohydrogen 2. An Approach to Environmentally Acceptable Technology, Miyake, J., Matsunaga, T., and San Pietro, A., Eds., Amsterdam: Pergamon, 2001, pp. 229–244.
Rocha, J., Barbosa, H.R., and Wijffels, R.H., Hydrogen production by photosynthetic bacteria: culture media, yields and efficiencies, in Biohydrogen 2. An Approach to Environmentally Acceptable Technology, Miyake, J., Matsunaga, T., and San Pietro, A., Eds., Amsterdam: Pergamon, 2001, pp. 3–32.
Tsygankov, A.A. and Laurinavichene, T.V., Influence of the degree and mode of light limitation on growth characteristics of the Rhodobacter capsulatus continuous cultures, Biotechn. Bioeng., 1996, vol. 51, pp. 605–612.
Tsygankov, A., Laurinavichene, T., Gogotov, I., Asada, Y., and Miyake, J., Switching over from light limitation to ammonium limitation of chemostat cultures of Rhodobacter capsulatus grown in different types of photobioreactors, J. Marine Biotechnol., 1996, vol. 4, pp. 43–46.
Gokce, A., Osturk, Y., Cakar, Z.P., and Yucel, M., Temperature resistant mutants of Rhodobacter capsulatus generated by direct evolution approach and effects of temperature resistance on hydrogen production, Int. J. Hydrogen Energy, 2012, vol. 37, pp. 15466–16472.
Merugu, R., Pratar Rudra, M.P., Badgu, N., Girisham, S., and Reddy, S.M., Factors influencing the production of hydrogen by the purple non-sulphur phototrophic bacterium Rhodopseudomonas acidophila KU001, Microb. Biotechnol., 2012, vol. 5, no. 6, pp. 674–678.
Laurinavichene, T.V., Tekucheva, D.N., Laurinavichius, K.S., Ghirardi, M.L., Seibert, M., and Tsygankov, A.A., Towards the integration of dark and photo fermentative waste treatment 1. Hydrogen photoproduction by purple bacterium Rhodobacter capsulatus using potential products of starch fermentation, Int. J. Hydrogen Energy, 2008, vol. 33, pp. 7020–7026.
Kapdan, I.K. and Kargi, F., Bio-hydrogen production from waste materials, Enzyme Microb. Technol. 2006, vol. 38, pp. 569–582.
Adessi, A. and De Philips, R., Photobioreactor design and illumination systems for H2 production with anoxygenic photosynthetic bacteria: a review, Int. J. Hydrogen Energy, 2014, vol. 39, pp. 3127–2141.
Hillmer, P. and Gest, H., H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: production and utilization of H2 by resting cells, J. Bacteriol., 1977, vol. 129, pp. 732–739.
Weaver, P.F., Lien, S., and Seibert, M., Photobiological production of hydrogen, Solar Energy, 1980, vol. 24, pp. 3–45.
Miyake, J. and Kawamura, S., Efficiency of light energy conversion to hydrogen by the photosynthetic bacterium Rhodobacter sphaeroides, Int. J. Hydrogen Energy, 1987, vol. 12, pp. 147–149.
Kern, M., Klipp, W., and Klemme, J.H., Increased nitrogenase-dependent H2 photoproduction by hup mutants of Rhodospirillum rubrum, App. Environ. Microbiol., 1994, vol. 60, pp. 1768–1774.
Fascetti, E. and Todini, O., Rhodobacter RV cultivation and hydrogen production in one- and two-stage chemostat, Appl. Microbiol. Biotechnol., 1995, vol. 20, pp. 300–305.
Zorin, N.A., Lissolo, T., Colbeau, A., and Vignais, P.M., Increased hydrogen photoproduction by Rhodobacter capsulatus strains deficient in uptake hydrogenase, J. Mar. Biotechnol., 1996, vol. 4, pp. 28–33.
Kohring, G.W., Fissler, J., Schirra, C., and Giffhorn, F., Hydrogen production from aromatic acids by liquid cultures and immobilized cells of Rhodopseudomonas palustris, in 11th World Hydrogen Energy Conf., 23–28 June, 1996, Germany, Stuttgart, 1996, pp. 2743–2748.
Franchi, E., Tosi, C., Scolla, G., Della Penna, G., Rodriguez, F., and Pedroni, P.M., Metabolically engineered Rhodobacter sphaeroides RV strains for improved biohydrogen photoproduction combined with disposal of food wastes, Marine Biotechnol., 2004, vol. 6, pp. 552–565.
Lee, J.Z., Klaus, D.M., Maness, P.C., and Spear, J.R., The effect of butyrate concentration on hydrogen production via photofermentation for use in a Martian habitat resource recovery process, Int. J. Hydrogen Energy, 2007, vol. 32, pp. 3301–3307.
Ryu, M.H., Hull, N.C., and Gomelsky, M., Metabolic engineering of Rhodobacter sphaeroides for improved hydrogen production, Int. J. Hydrogen Energy, 2014, vol. 39, pp. 6384–6390.
Eltsova, Z.A., Vasilieva, L.G., and Tsygankov, A.A., Hydrogen production by recombinant strains of Rhodobacter sphaeroides using modified photosynthetic apparatus, Appl. Biochem. Microbiol., 2010, vol. 46, no. 5, pp. 487–491.
Tsygankov, A. and Kosourov, S., Immobilization of photosynthetic microorganisms for efficient hydrogen production, in Microbial BioEnergy: Hydrogen Production. Advances in Photosynthesis and Respiration, vol. 38, Zannoni, D. and De Philippis, R., Eds., Springer, 2014, pp. 321–347.
Vincenzini, M., Materassi, R., Tredici, M.R., and Florenzano, G., Hydrogen production by immobilized cell. I. Light-dependent dissimilation of organic substances by Rhodopseudomonas palustris, Int. J. Hydrogen Energy, 1982, vol. 7, pp. 231–236.
Francou, N. and Vignais, P.M., Hydrogen production by Rhodopseudomonas capsulata cells entrapped in carrageenan beads, Biotechnol. Lett., 1984, vol. 6, pp. 639–644.
Hallenbeck, P.C., Immobilized microorganisms for hydrogen and ammonia production, Enzyme Microb. Technol., 1983, vol. 5, pp. 171–180.
Gosse, J.L., Engel, B.J., Rey, F.E., Harwood, C.S., Scriven, L.E., and Flickinger, M.C., Hydrogen production by photoreactive nanoporous latex coatings of nongrowing Rhodopseudomonas palustris CGA009, Biotechnol. Prog., 2007, vol. 23, pp. 124–130.
Laurinavichene, T.V., Fedorov, A.S., Ghirardi, M.L., Seibert, M., and Tsygankov, A.A., Demonstration of sustained hydrogen photoproduction by immobilized, sulfur-deprived Chlamydomonas reinhardtii cells, Int. J. Hydrogen Energy, 2006, vol. 31, pp. 659–667.
Tsygankov, A.A., Hirata, Y., Miyake, M., Asada, Y., and Miyake, J., Photobioreactor with photosynthetic bacteria immobilized on porous glass for hydrogen photoproduction, J. Ferment. Bioeng., 1994, vol. 77, pp. 575–578.
Tsygankov, A.A., Fedorov, A.S., Talipova, I.V., Miyaki, D., and Gogotov, I.N., Use of immobilized phototrophic microorganisms for water treatment and simultaneous production of hydrogen, Appl. Biochem. Microbiol., 1998, vol. 34, pp. 362–366.
Zagrodnik, R., Thiel, M., Seifert, K., Włodarczak M., and Łaniecki, M., Application of immobilized Rhodobacter sphaeroides bacteria in hydrogen generation process under semi-continuous conditions, Int. J. Hydrogen Energy, 2013, vol. 38, pp. 7632–7639.
Tsygankov, A., Hydrogen production by suspension and immobilized cultures of phototrophic microorganisms. Technological aspects, in Biohydrogen III. Renewable Energy System by Biological Solar Energy Conversion, Miyake, J., Igarashi, H., and Rogner, M., Eds., Elsevier, 2004, pp. 57–74.
Serebryakova, L.T. and Tsygankov, A.A., Two-stage system for hydrogen production by immobilized cyanobacterium Gloeocapsa alpicola CALU 743, Biotechnol. Prog., 2007, vol. 23, pp. 1106–1110.
Tekucheva, D.N., Laurinavichene, T.V., Seibert, M., and Tsygankov, A.A., Immobilized purple bacteria for light-driven H2 production from starch and potato fermentation effluent, Biotechnol. Prog., 2011, vol. 27, no. 5, pp. 568–599.
Fedorov, A., Tsygankov, A., Rao, K.K., and Hall, D.O., Hydrogen photoproduction by Rhodobacter capsulatus immobilized on polyurethane foam, Biotechnol. Lett., 1998, vol. 20, pp. 1007–1009.
Planchard, A., Mignot, L., Jouenne, T., and Junter, G.A., Photoproduction of molecular hydrogen by Rhodospirillum rubrum immobilized in composite agar layer/microporous membrane structures, Appl. Microbiol. Biotechnol., 1984, vol. 31, pp. 49–54.
von Felten, P., Zurrer, H., and Bachofen, R., Production of molecular hydrogen with immobilized cells of Rhodospirillum rubrum, Appl. Microbiol. Biotechnol., 1985, vol. 23, pp. 15–20.
Levin, D.B., Biohydrogen production: prospects and limitations to practical application—erratum, Int. J. Hydrogen Energy, 2004, vol. 29, pp. 1425–1426.
Levin, D.B., Pitt, L., and Love, M., Biohydrogen production: prospects and limitations to practical application, Int. J. Hydrogen Energy, 2004, vol. 29, pp. 173–185.
Tsygankov, A.A. and Khusnutdinova, A.N., Hydrogen metabolism in purple bacteria and its possible practical application, in Trudy Instituta mikrobiologii im. Vinogradskogo RAN (Proc. Winogradsky Inst. Microbiol.), vol. 16, Gal’chenko, V.F., Ed., Moscow: Maks Press, 2010, pp. 290–326.
Tsygankov, A.A. and Gogotov, I.N., Production of purple bacteria biomass, Appl. Biochem. Microbiol., 1990, vol. 26, pp. 819–824.
Tao, Y., He, Y., Wu, Y., Liu, F., Li, X., Zong, W., and Zhou, Z., Characteristics of a new photosynthetic bacterial strain for hydrogen production and its application in wastewater treatment, Int. J. Hydrogen Energy, 2008, vol. 33, pp. 963–973.
Zhu, H., Suzuki, T., Tsygankov, A., Asada, Y., and Miyake, J., Hydrogen production from tofu wastewater by Rhodobacter sphaeroides immobilized on agar gel, Int. J. Hydrogen Energy, 1999, vol. 24, pp. 305–310.
Tekucheva, D.N. and Tsygankov, A.A., Combined biological hydrogen-producing systems: a review, Appl. Biochem. Microbiol., 2012, vol. 48, no. 4, pp. 319–337.
Miyake, J., Mao, X.Y., and Kawamura, S., Hydrogen photoproduction from glucose by a co-culture of a photosynthetic bacteria and Clostridium butyricum, J. Ferment. Technol., 1984, vol. 62, pp. 531–535.
Asada, Y., Tokumoto, M., Aihara, Y., Oku, M., Ishimi, K., Wakayama, T., Miyake, J., Tomiyama, M., and Kohno, H., Hydrogen production by co-cultures of Lactobacillus and a photosynthetic bacterium, Rhodobacter sphaeroides RV, Int. J. Hydrogen Energy, 2006, vol. 31, pp. 1509–1513.
Kawaguchi, H., Hashimoto, K., Hirata, K., and Miyamoto, K., H2 production from algal biomass by mixed culture of Rhodobium marinum A-501 and Lactobacillus amylovorus, J. Biosci. Bioeng., 2001, vol. 91, pp. 277–282.
Yokoi, H., Mori, S., Hirose, J., Hayashi, S., and Takasaki, Y., H2 production from starch by mixed culture of Clostridium butyricum and Rhodobacter sp. M-19, Biotechnol. Lett., 1998, vol. 20, pp. 895–899.
Zhang, T., Liu, H., and Fang, H.H.P., Microbial analysis of a phototrophic sludge producing hydrogen from acidified wastewater, Biotechnol. Lett., 2002, vol. 24, pp. 1833–1837.
Claassen, P.A.M. and de Vrije, T., Non-thermal production of pure hydrogen from biomass: HYVOLU-TION, Int. J. Hydrogen Energy, 2006, vol. 31, pp. 1416–1423.
Kim, M.S., Lee, T.J., Yoon, Y.S., Lee, I.G., and Moon, K.W., Hydrogen production from food processing wastewater and sewage sludge by anaeroibic dark fermentation combined with photo-fermentation, in Biohydrogen II. An Approach to Environmentally Acceptable Technology, Miyake, J., Matsunaga, T., and San Pietro, A., Eds., Amsterdam: Pergamon, 2001, pp. 263–272.
Kulichevskaya, I.S., Gusev, V.S., Gorlenko, M.V., Liesack, W., and Dedysh, S.N., Rhodoblastus sphagnicola sp. nov., a novel acidophilic purple non-sulfur bacterium from Sphagnum peat bog, Int. J. Syst. Evol. Microbiol., 2006, vol. 56, pp. 1397–1402.
Laurinavichene, T.V., Belokopytov, B.F., Laurinavichius, K.S., Tekucheva, D.N., Ghirardi, M.L., Seibert, M., and Tsygankov, A.A., Towards the integration of darkand photo-fermentative waste treatment. 3. Potato as substrate for sequential dark fermentation and light-driven H2 production, Int. J. Hydrogen Energy, 2010, vol. 35, pp. 8536–8543.
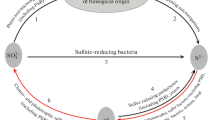
Maeda, I., Mizoguchi, T., Miura, Y., Yagi, K., Shioji, N., and Miyasaka, H., Influence of sulfatereducing bacteria on outdoor hydrogen production by photosynthetic bacterium with seawater, Curr. Microbiol., 2000, vol. 40, pp. 210–213.
Laurinavichene, T., Laurinavichius, K., Belokopytov, B., Laurinavichyute, D., and Tsygankov, A., Influence of sulfate-reducing bacteria, sulfide and molybdate on hydrogen photoproduction by purple nonsulfur bacteria, Int. J. Hydrogen Energy, 2013, vol. 38, pp. 5545-5554.
Golomysova, A., Gomelsky, M., and Ivanov, P.S., Flux balance analysis of photoheterotrophic growth of purple nonsulfur bacteria relevant to biohydrogen production, Int. J. Hydrogen Energy, 2010, vol. 35, no. 23, pp. 12751–12760.
Kars, G., Gunduz, U., Rakhely, G., Yucel, M., Eroglu, I., and Kovacs, K.L., Improved hydrogen production by uptake hydrogenase deficient mutant strain of Rhodobacter sphaeroides OU001, Int. J. Hydrogen Energy, 2008, vol. 33, pp. 3056–3060.
Kars, G., Rakhely, G., and Kovacs, K.L., Evaluation of hydrogen production by Rhodobacter sphaeroides O.U.001 and its hupSL deficient mutant using acetate and malate as carbon sources, Int. J. Hydrogen Energy, 2009, vol. 34, pp. 2184–2190.
Kim, M.S., Baek, J.S., and Lee, J., Comparison of H2 accumulation by Rhodobacter sphaeroides KD131 and its uptake hydrogenase and PHB synthase deficient mutant, Int. J. Hydrogen Energy, 2006, vol. 31, pp. 121–127.
Li, R.Y. and Fang, H.H.R., Hydrogen production characteristics of photoheterotrophic Rubrivivax gelatinosus L31, Int. J. Hydrogen Energy, 2008, vol. 33, pp. 974–980.
Khusnutdinova, A., Ovchenkova, E., Khristova, A., Laurinavichene, T., Shastik, E., Liu, J., and Tsygankov, A., New tolerant strains of purple nonsulfur bacteria for hydrogen production in a two-stage integrated system, Int. J. Hydrogen Energy, 2012, vol. 37, pp. 8820–8827.
Wang, Z., Hu, X., Liao, Q., Ye, J., Zhang, B., and Yin, Y., Identification, culture characteristics and hydrogen-producing ability analysis of several purple non-sulfur bacterial strains, Chinese J. Appl. Environ. Biol., 2009, vol. 15, pp. 120–124.
Kim, E.J., Kim, J.S., Kim, M.S., and Lee, J.K., Effect of changes in the level of light harvesting complexes of Rhodobacter sphaeroides on the photoheterotrophic production of hydrogen, Int. J. Hydrogen Energy, 2006, vol. 31, pp. 531–538.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Tsygankov, A.N. Khusnutdinova, 2015, published in Mikrobiologiya, 2015, Vol. 84, No. 1, pp. 3–26.
Rights and permissions
About this article
Cite this article
Tsygankov, A.A., Khusnutdinova, A.N. Hydrogen in metabolism of purple bacteria and prospects of practical application. Microbiology 84, 1–22 (2015). https://doi.org/10.1134/S0026261715010154
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261715010154