Abstract
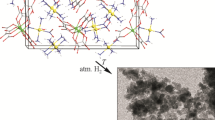
The [(Pt(NH3)4)x(Pd(NH3)4)1–x]CrO4 single-phase solid solution (x = 0.5-0.6) is prepared by the cocrystallization of [Pt(NH3)4](NO3)2 and [Pd(NH3)4](NO3)2 aqueous solutions with ammoniac (NH4)2CrO4. The structure and parameters of the tetragonal (I41/amd, Z = 4) unit cell (a = 7.3091(1) Å, c = 15.2720(6) Å) of this compound and the molar fraction of Pt (x = 0.568(7)) are determined using the single-crystal X-ray diffraction method. The unit cell parameters of the studied crystal are additionally refined by an original method (a = 7.3207(9) Å, c = 15.2527(19) Å, V = 817.4(3) Å3) and are further utilized to refine also the molar fraction of Pt using Zen′s law (x = 0.57(1)). A single crystal of [(Pt(NH3)4)0.57(Pd(NH3)4)0.43]CrO4 and two samples of the synthesized product selected from the total mass are thermally decomposed in hydrogen. A study of thermolysis products by XRD, scanning and transmission electron microscopy methods demonstrates the formation of a Pt0.49Pd0.38Cr0.13 nanoalloy included in the Cr2O3 matrix in the form of blocks (~1 µm) and smaller (>2 nm) spherical formations.







Similar content being viewed by others
REFERENCES
D. P. Domonov, S. I. Pechenyuk, A. T. Belyaevskii, and K. V. Yusenko. Formation of nanostructured carbon from [Ni(NH3)6]3[Fe(CN)6]2. Nanomaterials, 2020, 10(2), 389. https://doi.org/10.3390/nano10020389
D. P. Domonov, S. I. Pechenyuk, Y. P. Semushina, and G. I. Kadyrova. Solid-state transformations by thermal decomposition of [Co(en)3][Fe(C2O4)3] in an inert atmosphere. Thermochim. Acta, 2020, 687, 178578. https://doi.org/10.1016/j.tca.2020.178578
A. V. Zadesenets, E. Y. Filatov, P. E. Plyusnin, T. I. Asanova, I. V. Korolkov, I. A. Baidina, E. V. Shlyakhova, I. P. Asanov, and S. V. Korenev. Complex salts of Pd(II) and Pt( II) with Co(II) and Ni(II) aqua-cations as single-source precursors for bimetallic nanoalloys and mixed oxides. New J. Chem., 2018, 42(11), 8843-8850. https://doi.org/10.1039/c8nj00956b
D. Domonov, S. Pechenyuk, Y. Semushina, and K. Yusenko. Solid-state transformations in inner coordination sphere of [Co(NH3)6][Fe(C2O4)3]·3H2O as a route to access catalytically active Co–Fe materials. Materials, 2019, 12(2), 221. https://doi.org/10.3390/ma12020221
D. Vasilchenko, P. Topchiyan, S. Berdyugin, E. Filatov, S. Tkachev, I. Baidina, V. Komarov, E. Slavinskaya, A. Stadnichenko, and E. Gerasimov. Tetraalkylammonium salts of platinum nitrato complexes: isolation, structure, and relevance to the preparation of PtOx/CeO2 catalysts for low-temperature CO oxidation. Inorg. Chem., 2019, 58(9), 6075-6087. https://doi.org/10.1021/acs.inorgchem.9b00370
E. Filatov, V. Lagunova, D. Potemkin, N. Kuratieva, A. Zadesenets, P. Plyusnin, A. Gubanov, and S. Korenev. Tetraammineplatinum(II) and tetraamminepalladium(II) chromates as precursors of metal oxide catalysts. Chem. - Eur. J., 2020, 26(19), 4341-4349. https://doi.org/10.1002/chem.201905391
D. I. Potemkin, E. Y. Filatov, A. V. Zadesenets, V. N. Rogozhnikov, E. Y. Gerasimov, P. V. Snytnikov, S. V. Korenev, and V. A. Sobyanin. Bimetallic Pt–Co/η-Al2O3/FeCrAl wire mesh composite catalyst prepared via double complex salt [Pt(NH3)4][Co(C2O4)2(H2O)2]·2H2O decomposition. Mater. Lett., 2019, 236, 109-111. https://doi.org/10.1016/j.matlet.2018.10.097
D. Vasilchenko, P. Topchiyan, I. Baidina, I. Korolkov, E. Filatov, V. Zvereva, P. Plyusnin, E. Slavinskaya, and E. Gerasimov. Double complex salts containing [Pt(NO3)6]2- anion and Rh(III) complex cations: Synthesis, structure and utilisation for preparing (Rh–Pt)/CeO2 catalysts. J. Mol. Struct., 2020, 1211, 128108. https://doi.org/10.1016/j.molstruc.2020.128108
Y. Duan, Z.-Y. Yu, L. Yang, L.-R. Zheng, C.-T. Zhang, X.-T. Yang, F.-Y. Gao, X.-L. Zhang, X. Yu, R. Liu, H.-H. Ding, C. Gu, X.-S. Zheng, L. Shi, J. Jiang, J.-F. Zhu, M.-R. Gao, and S.-H. Yu. Bimetallic nickel-molybdenum/tungsten nanoalloys for high-efficiency hydrogen oxidation catalysis in alkaline electrolytes. Nat. Commun., 2020, 11(1), 4789. https://doi.org/10.1038/s41467-020-18585-4
V. Lagunova, E. Filatov, P. Plyusnin, G. Kostin, A. Urlukov, D. Potemkin, and S. Korenev. Metal-oxide catalysts for CO TOX and PROX processes in the Pt–Cr/Mo/W systems. Int. J. Hydrogen Energy, 2023, 48(64), 25133-25143. https://doi.org/10.1016/j.ijhydene.2022.09.086
E. Y. Filatov, Y. P. Semushina, and A. N. Gosteva. Obtaining and catalytic properties investigation of the products of double-complex salts [Cr(ur)6][M(L)6] thermal oxidation (M = Co, Fe; L = CN−, 1/2C2O42− ). J. Therm. Anal. Calorim., 2018, 134(1), 355-361. https://doi.org/10.1007/s10973-018-7230-y
E. Filatov, V. Lagunova, D. Potemkin, and S. Korenev. Composite of Pd or Pt with chromium oxide (III) as catalysts in CO PROX and CO TOX processes. 11th International Conference on Nanomaterials - Research and Application (NANOCON 2019), Brno, Czech Republic, October 16-18, 2019. Ostrava, Czech Republic: TANGER, 2020, 180-185.
P. S. Serebrennikova, V. Y. Komarov, A. S. Sukhikh, S. P. Khranenko, A. V. Zadesenets, S. A. Gromilov, and K. V. Yusenko. [NiEn3](MoO4)0.5(WO4)0.5 co-crystals as single-source precursors for ternary refractory Ni–Mo–W Alloys. Nanomaterials, 2021, 11(12), 3272. https://doi.org/10.3390/nano11123272
P. S. Serebrennikova and S. A. Gromilov. Study of [Pd(NH3)4](MoO4)x(CrO4)1–x solid solutions and products of their thermolysis. J. Struct. Chem., 2022, 63(11), 1856-1871. https://doi.org/10.1134/s0022476622110166
V. I. Lagunova, E. Y. Filatov, P. E. Plyusnin, and S. V. Korenev. In situ and ex situ studies of tetrammineplatinum(II) chromate thermolysis. Russ. J. Inorg. Chem., 2020, 65(10), 1566-1570. https://doi.org/10.1134/s0036023620100150
A. N. Gosteva, P. E. Plyusnin, Y. P. Semushina, S. I. Pechenyuk, E. Y. Filatov, and O. Y. Kyrtova. The thermal behavior of double complex compounds with the cation [Cr(ur)6]3+ in a reducing atmosphere. J. Therm. Anal. Calorim., 2018, 134(1), 253-260. https://doi.org/10.1007/s10973-018-7428-z
K. V. Yusenko, S. I. Pechenyuk, E. S. Vikulova, Y. P. Semushina, I. A. Baidina, and E. Y. Filatov. Isostructurality and thermal properties in the series of double complex salts [M1(NH3)6][M2(C2O4)3]·3H2O (M1 = Co, Ir, M2 = Fe, Cr). J. Struct. Chem., 2019, 60(7), 1062-1071. https://doi.org/10.1134/s0022476619070060
G. A. Kostin, P. E. Plyusnin, E. Y. Filatov, N. V. Kuratieva, A. A. Vedyagin, and D. B. Kal′nyi. Double complex salts [PdL4][RuNO(NO2)4OH] (L = NH3, Py) synthesis, structure and preparation of bimetallic metastable solid solution Pd0.5Ru0.5. Polyhedron, 2019, 159, 217-225. https://doi.org/10.1016/j.poly.2018.11.065
K. V. Yusenko, S. Khandarkhaeva, M. Bykov, T. Fedotenko, M. Hanfland, A. Sukhikh, S. A. Gromilov, and L. S. Dubrovinsky. Face-centered cubic refractory alloys prepared from single-source precursors. Materials, 2020, 13(6), 1418. https://doi.org/10.3390/ma13061418
Y. Laligant. On the first palladium chromate: crystal structure of Pd(NH3)4(CrO4). Eur. J. Solid State Inorg. Chem., 1993, 30(6), 681-688.
P. S. Serebrennikova and S. A. Gromilov. Calibration of the goniometer equatorial circumference and the refinement of unit cell parameters of [Pt(NH3)4]MO4 (M = Cr, Mo, W). J. Struct. Chem., 2022, 63(11), 1820-1830. https://doi.org/10.1134/s0022476622110129
E. N. Tupikova, I. A. Platonov, and T. N. Lykova. Hydrothermal synthesis of nanosize phases based on non-ferrous and noble metals. AIP Confer. Proc., 2016, 1724(1), 020052. https://doi.org/10.1063/1.4945172
J. E. Van Dam, P. C. M. Gubbens, and G. J. Van den Berg. The magnetic susceptibility of some Pd–Cr and Pt–Cr alloys. Physica, 1973, 70(3), 520-546. https://doi.org/10.1016/0031-8914(73)90361-3
C.-Y. Hu, Y.-F. Chiu, C.-C. Tsai, C.-C. Huang, K.-H. Chen, C.-W. Peng, C.-M. Lee, M.-Y. Song, Y.-L. Huang, S.-J. Lin, and C.-F. Pai. Toward 100% spin–orbit torque efficiency with high spin–orbital hall conductivity Pt–Cr alloys. ACS Appl. Electron. Mater., 2022, 4(3), 1099-1108. https://doi.org/10.1021/acsaelm.1c01233
C. J. Lee, J. Park, J. M. Kim, Y. Huh, J. Y. Lee, and K. S. No. Low-temperature growth of carbon nanotubes by thermal chemical vapor deposition using Pd, Cr, and Pt as co-catalyst. Chem. Phys. Lett., 2000, 327(5/6), 277-283. https://doi.org/10.1016/s0009-2614(00)00877-0
M. Fernandezgarcia. Behavior of bimetallic Pd–Cr/Al2O3 and Pd–Cr/(Ce,Zr)Ox/Al2O3 catalysts for CO and NO elimination. J. Catal., 2003, 214(2), 220-233. https://doi.org/10.1016/s0021-9517(02)00145-8
M. Min and H. Kim. Performance and stability studies of PtCr/C alloy catalysts for oxygen reduction reaction in low temperature fuel cells. Int. J. Hydrogen Energy, 2016, 41(39), 17557-17566. https://doi.org/10.1016/j.ijhydene.2016.07.175
D. Kaewsai, S. Yeamdee, S. Supajaroon, and M. Hunsom. ORR activity and stability of PtCr/C catalysts in a low temperature/pressure PEM fuel cell: Effect of heat treatment temperature. Int. J. Hydrogen Energy, 2018, 43(10), 5133-5144. https://doi.org/10.1016/j.ijhydene.2018.01.101
A. M. Mebed, E. F. A. Zeid, and A. M. Abd-Elnaiem. Synthesis and thermal treatment of Pd–Cr@carbon for efficient oxygen reduction reaction in proton-exchange membrane fuel cells. J. Inorg. Organomet. Polym. Mater., 2021, 31(9), 3772-3779. https://doi.org/10.1007/s10904-021-01991-6
K. Peng, N. Bhuvanendran, S. Ravichandran, W. Zhang, Q. Ma, L. Xing, Q. Xu, L. Khotseng, and H. Su. Carbon supported PtPdCr ternary alloy nanoparticles with enhanced electrocatalytic activity and durability for methanol oxidation reaction. Int. J. Hydrogen Energy, 2020, 45(43), 22752-22760. https://doi.org/10.1016/j.ijhydene.2020.06.101
J.-C. Zhao, M. R. Jackson, L. A. Peluso, and L. N. Brewer. A diffusion-multiple approach for mapping phase diagrams, hardness, and elastic modulus. JOM, 2002, 54(7), 42-45. https://doi.org/10.1007/bf02700985
A. V. Alexeev and S. A. Gromilov. X-Ray Diffraction study of micro amounts of polycrystalline samples. J. Struct. Chem., 2010, 51(4), 744-757. https://doi.org/10.1007/s10947-010-0110-3
C. Prescher and V. B. Prakapenka. DIOPTAS: a program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res., 2015, 35(3), 223-230. https://doi.org/10.1080/08957959.2015.1059835
B. H. Toby and R. B. Von Dreele. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr., 2013, 46(2), 544-549. https://doi.org/10.1107/s0021889813003531
APEX3 V.2019.1-0, SAINT V.8.40A and SADABS-V.2016/2. Madison, Wisconsin, USA: Bruker Advanced X-ray Solutions, 2016-2019.
G. M. Sheldrick. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053273314026370
G. M. Sheldrick. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053229614024218
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr., 2009, 42(2), 339-341. https://doi.org/10.1107/s0021889808042726
V. I. Lysoivan. Izmerenie parametrov elementarnoi yacheyki na odnokristal′nom spektrometre (Measurement of Unit Cell Parameters on a Single-Crystal Spectrometer). Novosibirsk, Russia: Nauka, 1982. [In Russian]
OriginPro. Version 2022b. Northampton, MA, USA: OriginLab Corporation, 2022.
K. V. Yusenko, S. Riva, P. A. Carvalho, M. V. Yusenko, S. Arnaboldi, A. S. Sukhikh, M. Hanfland, and S. A. Gromilov. First hexagonal close packed high-entropy alloy with outstanding stability under extreme conditions and electrocatalytic activity for methanol oxidation. Scr. Mater., 2017, 138, 22-27. https://doi.org/10.1016/j.scriptamat.2017.05.022
K. V. Yusenko, S. A. Martynova, S. Khandarkhaeva, T. Fedotenko, K. Glazyrin, E. Koemets, M. Bykov, M. Hanfland, K. Siemensmeyer, A. Smekhova, S. A. Gromilov, and L. S. Dubrovinsky. High compressibility of synthetic analogous of binary iridium–ruthenium and ternary iridium–osmium–ruthenium minerals. Materialia, 2020, 14, 100920. https://doi.org/10.1016/j.mtla.2020.100920
Inorganic Crystal Structure Database. D–1754. Eggenstein–Leopoldshafen, Germany: FIZ Karlsruhe, https://icsd.fiz-karlsruhe.de.
Powder Diffraction File. PDF-2. Newtown, USA: International Centre for Diffraction Data, 2021, https://www.icdd.com.
Funding
The synthesis of the samples was funded by the Russian Science Foundation (project No. 22-23-00672, https://rscf. ru/project/22-23-00672/). The authors thank the Ministry of Science and Higher Education of the Russian Federation and the Priority 2030 program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 9, 116969.https://doi.org/10.26902/JSC_id116969
Supplementary material
Rights and permissions
About this article
Cite this article
Serebrennikova, P.S., Lagunova, V.I., Zadesenets, A.V. et al. PREPARATION OF NANOALLOYS OF THE Pt–Pd–Cr SYSTEM UNDER THERMAL DECOMPOSITION OF [(Pt(NH3)4)x(Pd(NH3)4)1–x]CrO4 COMPLEX SALTS. J Struct Chem 64, 1686–1701 (2023). https://doi.org/10.1134/S0022476623090123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623090123