Abstract
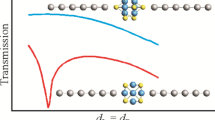
Electron transport through molybdenum chalcogenide cluster molecules Mo6Q8 (Q = S, Se, and Te) located between two 1D monoatomic aluminum chains is studied using non-equilibrium Green’s function. Electron transport depends on the cluster orientation relative to electrodes, the type of chalcogen atoms, and chemical the bonding between terminal aluminum and chalcogen atoms. Distances characterized by the maximum transport properties are determined from the scan of gaps between terminal atoms of the electrode and the molecule. Conductances of Mo6Se8 and Mo6Te8 clusters are shown to be comparable at the Fermi level, and in some cases, can surpass the conductance of the Mo6S8 cluster.





Similar content being viewed by others
REFERENCES
J. Moreland and J. W. Ekin. Electron tunneling experiments using Nb–Sn «break» junctions. J. Appl. Phys., 1985, 58(10), 3888-3895. https://doi.org/10.1063/1.335608
C. J. Muller, J. M. van Ruitenbeek, and L. J. de Jongh. Experimental observation of the transition from weak link to tunnel junction. Phys. C, 1992, 191(3/4), 485-504. https://doi.org/10.1016/0921-4534(92)90947-b
D. Xiang, H. Jeong, T. Lee, and D. Mayer. Mechanically controllable break junctions for molecular electronics. Adv. Mater., 2013, 25(35), 4845-4867. https://doi.org/10.1002/adma.201301589
P. Gehring, J. M. Thijssen, and H. S. J. van der Zant. Single-molecule quantum-transport phenomena in break junctions. Nat. Rev. Phys., 2019, 1(6), 381-396. https://doi.org/10.1038/s42254-019-0055-1
R. H. M. Smit, Y. Noat, C. Untiedt, N. D. Lang, M. C. van Hemert, and J. M. van Ruitenbeek. Measurement of the conductance of a hydrogen molecule. Nature, 2002, 419(6910), 906-909. https://doi.org/10.1038/nature01103
M. H. Lee, G. Speyer, and O. F. Sankey. Electron transport through single alkane molecules with different contact geometries on gold. Phys. Status Solidi, 2006, 243(9), 2021-2029. https://doi.org/10.1002/pssb.200666804
E. M. Dief, P. J. Low, I. Díez-Pérez, and N. Darwish. Advances in single-molecule junctions as tools for chemical and biochemical analysis. Nat. Chem., 2023, 15(5), 600-614. https://doi.org/10.1038/s41557-023-01178-1
J. Shin, J.S. Eo, T. Jeon, T. Lee, and G. Wang. Advances of various heterogeneous structure types in molecular junction systems and their charge transport properties. Adv. Sci., 2022, 9(30), 2202399. https://doi.org/10.1002/advs.202202399
I. Popov, T. Yang, S. Berber, G. Seifert, and D. Tománek. Unique structural and transport properties of molybdenum chalcohalide nanowires. Phys. Rev. Lett., 2007, 99(8), 085503. https://doi.org/10.1103/physrevlett.99.085503
I. Popov, S. Gemming, and G. Seifert. Structural and electronic properties of Mo6S8 clusters deposited on a Au(111) surface investigated with density functional theory. Phys. Rev. B, 2007, 75(24), 245436. https://doi.org/10.1103/physrevb.75.245436
M. R. Ryzhikov and S. G. Kozlova. Electron transport through the Mo6S8 molecule in the electrode–cluster–electrode system: effect of the cluster remoteness and orientation relative to the electrodes. J. Struct. Chem., 2022, 63(11), 1745-1750. https://doi.org/10.1134/s0022476622110038
N. Chevreau and D. C. Johnson. Preparation and physical properties of BiMo6S8 and SbMo6S8. J. Solid State Chem., 1986, 61(3), 347-353. https://doi.org/10.1016/0022-4596(86)90042-3
P. Gougeon and P.Gall. Direct solid-state synthesis of the superconductor NaMo6Se8: Single-crystal structure, electrical and magnetic properties. Cambridge, England: Cambridge Open Engage, ChemRxiv, 2022. https://doi.org/10.26434/chemrxiv-2022-7zf68
G. J. Miller and M. Smith. Hexamolybdenum octatelluride, Mo6Te8. Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1998, 54(6), 709-710. https://doi.org/10.1107/s0108270197017812
ADF 2022.102. Amsterdam, The Netherlands: SCM, Theoretical Chemistry, Vrije Universiteit, 2022, https://www.scm.com (accessed Jan 10, 2023).
A. D. Becke. Density-functional exchange-energy approximation with correct asymptotic behavior. Physical Review A, 38(6). 3098-3100. https://doi.org/10.1103/physreva.38.3098
J. P. Perdew, K. Burke, and M. Ernzerhof. Generalized gradient approximation made simple. Phys. Rev. Lett., 1996, 77(18), 3865-3868. https://doi.org/10.1103/physrevlett.77.3865
E. Van Lenthe and E. J. Baerends. Optimized Slater-type basis sets for the elements 1-118. J. Comput. Chem., 2003, 24(9), 1142-1156. https://doi.org/10.1002/jcc.10255
E. van Lenthe, A. Ehlers, and E.-J. Baerends. Geometry optimizations in the zero order regular approximation for relativistic effects. J. Chem. Phys., 1999, 110(18), 8943-8953. https://doi.org/10.1063/1.478813
S. Datta. Quantum Transport: Atom to Transistor. New York, USA: Cambridge University Press, 2005.
C. J. O. Verzijl and J. M. Thijssen. DFT-based molecular transport implementation in ADF/BAND. J. Phys. Chem. C, 2012, 116(46), 24393-24412. https://doi.org/10.1021/jp3044225
R. Li, J. Zhang, S. Hou, Z. Qian, Z. Shen, X. Zhao, and Z. Xue. A corrected NEGF+DFT approach for calculating electronic transport through molecular devices: Filling bound states and patching the non-equilibrium integration. Chem. Phys., 2007, 336(2/3), 127-135. https://doi.org/10.1016/j.chemphys.2007.06.011
BAND 2022.102. Amsterdam, The Netherlands: SCM, Theoretical Chemistry, Vrije Universiteit, 2022, https://www.scm.com (accessed Jan 10, 2023).
C. J. Lambert. Basic concepts of quantum interference and electron transport in single-molecule electronics. Chem. Soc. Rev., 2015, 44(4), 875-888. https://doi.org/10.1039/c4cs00203b
M. Strange, I. S. Kristensen, K. S. Thygesen, and K. W. Jacobsen. Benchmark density functional theory calculations for nanoscale conductance. J. Chem. Phys., 2008, 128(11), 114714. https://doi.org/10.1063/1.2839275
R. F. W. Bader. Atoms in Molecules. A Quantum Theory. New York: Clarendon, 1990.
E. Espinosa, I. Alkorta, J. Elguero, and E. Molins. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–Y systems. J. Chem. Phys., 2002, 117(12), 5529-5542. https://doi.org/10.1063/1.1501133
CRC Handbook of Chemistry and Physics, 95th ed. / Eds. W.M. Haynes, D.R. Lide, T.J. Bruno. Boca Raton, FL, USA: CRC Press, 2014-2015.
D. O. Arentov, M. R. Ryzhikov, and S. G. Kozlova. The role of quadruple bonding in the electron transport through a dimolybdenum tetraacetate molecule. Molecules, 2022, 27(20), 6912. https://doi.org/10.3390/molecules27206912
Funding
The work was supported by the Russian Science Foundation (grant No. 22-23-00245).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 8, 114895.https://doi.org/10.26902/JSC_id114895
Rights and permissions
About this article
Cite this article
Ryzhikov, M.R., Kozlova, S.G. Electron Transport Through Octahedral Molybdenum Chalcogenide Clusters in Electrode–Cluster–Electrode Systems. J Struct Chem 64, 1525–1531 (2023). https://doi.org/10.1134/S0022476623080164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623080164