Abstract
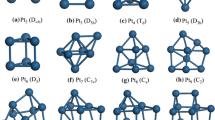
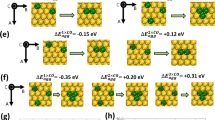
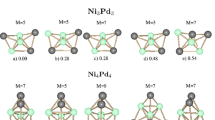
Bimetallic alloys are considered to be a promising type of catalysts with improved activity and selectivity that are distinct from those of the corresponding pure nanoclusters [1-4]. Using first principles density functional calculations, we study the structures and energies of Al n Pt bimetallic clusters up to 13 atoms. If platinum, nickel, and other transition metal catalysts are particularly important in the catalysis of hydrogen, hydrogen adsorption on a metal surface is an important step in the catalytic reaction. Because of large exothermic energy changes and relatively small activation energies, Al7Pt and Al12Pt could serve as highly efficient and low-cost catalysts for the hydrogen dissociation. To clarify this assumption and achieve a good understanding, the H2 adsorption and dissociation over bimetallic AlPt clusters are systematically investigated in our work.
Similar content being viewed by others
References
Y. Mizukoshi, T. Fujimoto, Y. Nagata, R. Oshima, and Y. Maeda, J Phys. Chem. B, 104, 6028–6032 (2000).
K. R. Harikumar, S. Ghosh, and C. N. R. Rao, J Phys. Chem. A, 101, 536–540 (1997).
A. C. Templeton, W. P. Wuelfing, and R. W. Murray, Acc. Chem. Res., 33, 27–36 (2000).
R. L. Whetten, M. N. Shafigullin, J. T. Khoury, T. G. Schaaff, I. Vezmar, M. M. Alvarez, and A. Wilkinson, Acc. Chem. Res., 32, 397–406 (1999).
C. H. Christensen and J. K. Norskov, J. Chem. Phys., 128, 182503-182503-8 (2008).
F. Tao, M. E. Grass, Y. W. Zhang, D. R. Butcher, J. R. Renzas, Z. Liu, J. Y. Chung, B. S. Mun, M. Salmeron, and G. A. Somorjai, Science, 322, 932–934 (2008).
Y. G. Ma and P. B. Balbuena, J Phys. Chem. C, 112, 14520 (2008).
O. M. Lovvik and S. M. Opalka, Surf. Sci., 602, 2840–2844 (2008).
V. Soto-Verdugo and H. Metiu, Surf. Sci., 601, No. 23, 5332–5339 (2007).
W. Z. Li, C. H. Liang, W. J. Zhou, J. S. Qiu, Z. H. Zhou, G. Q. Sun, and Q. Xin, J Phys. Chem. B, 107, No. 26, 6292–6299 (2003).
P. K. Babu, Y. Y. Tong, H. S. Kim, and A. Wieckowski, J Electroanal. Chem., 524, 157–167 (2002).
H. Q. Li, Q. Xin, W. Z. Li, Z. H. Zhou, L. H. Jiang, S. H. Yang, and G. Q. Sun, Chem. Commun., No. 23, 2776/2777 (2004).
U. A. Paulus, A. Wokaun, G. G. Scherer, T. J. Schmidt, V. Stamenkovic, V. Radmilovic, N. M. Markovic, and P. N. Ross, J Phys. Chem. B, 106, No. 16, 4181–4191 (2002).
O. Savadogo, K. Lee, K. Oishi, S. Mitsushima, N. Kamiya, and K. I. Ota, Electrochem. Commun., 6, No. 2, 105–109 (2004).
J. L. Fernandez, D. A. Walsh, and A. J. Bard, J Am. Chem. Soc., 127, No. 1, 357–365 (2005).
P. C. Jennings, B. G. Pollet, and R. L. Johnston, J Phys. Chem. C, 116, 15241–15250 (2012).
D. L. Peng, T. Hihara, and K. Sumiyama, J Mag. Mag. Mat., 277, No. 1, 201–208 (2004).
Y. Benguedouar, N. Keghouche, and J Belloni, Mater. Sci. Eng., B, t177, No. 1, 27–33 (2012).
Q. Ge, C. Song, and L. Wang, J Comput. Mater. Sci., 35, No. 3, 247–253 (2006).
Y. W. Huang, T. Y. Chou, G. Y. Yu, and S. L. Lee, J Phys. Chem. C, 115, No. 18, 9105–9116 (2011).
W. Chen, D. Schmidt, W. F. Schneider, and C. Wolverton, J Phys. Chem. C, 115, No. 36, 17915–17924 (2011).
Y. Ishikawa, R. R. Diaz-Morales, A. Perez, M. J. Vilkas, and C. R. Cabrera, J Chem. Phys. Lett., 411, No. 4, 404–410 (2005).
M. J. Piotrowski, P. Piquini, Z. H. Zeng, and J. L. F. Da Silva, J Phys. Chem. C, 116, 20540–20549 (2012).
P. Tarakeshwar, T. J. Dhilip Kumar, and N. Balakrishnan, J Chem. Phys., 130, 114301-1-9 (2009).
H. S. Huang, X. M. Wang, D. Q. Zhao, L. F. Wu, X. W. Huang, and Y. C. Li, Acta Phys. Sin., 61, No. 7, 073101 (2012).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakasuji, M. Hada, M. Ehara, K. Toyota, R., Fukuda R. Hasegawa, M. J. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. R. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, J. A. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03 (Revision Co2), Gaussian. Inc., Pittsburgh., PA (2003).
A. D. Becke, J Chem. Phys., 98, 5648–1 (1993).
C. Peng, P. Y. Ayala, H. B. Schlegel, and M. J. Frisch, J Comput. Chem., 17, No. 1, 49–56 (1996).
C. Gonzalez and H. B. Schlegel, J Chem. Phys., 90, 2154 (1989)
C. Gonzalez and H. B. Schlegel, J Phys. Chem., 94, No. 14, 5523–5527 (1990).
F. C. Chuang, C. Z. Wang, and K. H. Ho, Phys. Rev. B, 73, No. 12, 125431–1 (2006)
A. Aguado and J. M. Lopez, J Chem. Phys., 130, 064704–1 (2009)
B. K. Rao and P. Jena, J Chem. Phys., 111, 1890 (1999)
R. R. Zope and T. Baruah, Phys. Rev. A, 64, No. 5, 053202–1 (2001).
X. H. Cheng, D. J. Ding, Y. G. Yu, and M. X. Jin, Chin. J Chem. Phys., 25, 169–176 (2012)
M. X. Chen, X. H. Yan, and S. H. Wei, J Phys. Chem. A, 111, No. 35, 8659–8662 (2007)
L. Guo, J Alloys Compd., 466, 463–470 (2008)
V. A. Gorbunov and L. I. Kurkina, Bull. Russ. Acad. Sci.: Phys., 72, No. 4, 515–519 (2008).
R. Jin, S. Zhang, Y. Zhang, S. Huang, P. Wang, and H. Tian, Int. J Hydrogen Energy, 36, No. 15, 9069–9078 (2011).
W. Lu, J Zhao, Z. Zhou, S. B. Zhang, and Z. Chen, J Comput. Chem., 30, No. 15, 2509–2514 (2009).
Q. L. Lu and J. G. Wan, J Chem. Phys., 132, 224308–1 (2010).
R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press, New York (1989).
R. G. Parr and R. G. Pearson, J Am. Chem. Soc., 105, No. 26, 7512–7516 (1983).
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 56, No. 4, pp. 656-666, July-August, 2015. Original article submitted January 12, 2014.
Rights and permissions
About this article
Cite this article
An, X., Guo, L., Ren, N. et al. A density functional theory analysis of the molecular hydrogen dissociation on Al n Pt (n = 1-12) clusters. J Struct Chem 56, 608–618 (2015). https://doi.org/10.1134/S0022476615040022
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476615040022