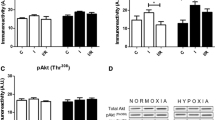
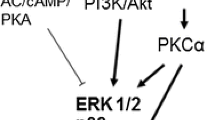
Abstract
The cardioprotective effect of chronic hypoxia (CH) is associated with the activation of inducible nitric oxide synthase (iNOS). Reactive oxygen species (ROS) are involved in the development of CH-induced cardiac tolerance to ischemia/reperfusion. The infarct-limiting effect of CH depends on the opening of mitochondrial ATP-sensitive potassium (mitoKATP) channels. Protein kinase C δ and ε isoforms are involved in the cardioprotective effect of adaptation to hypoxia. CH increases the expression of phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2), Ca2+/calmodulin-dependent protein kinase II (CaMKII), phosphorylated p38 (p-p38), phosphorylated AMP-activated protein kinase (p-AMPK), as well as hexokinase-1 (HK1) and hexokinase-2 (HK2). ERK1/2 and mitogen-activated protein kinases (MEK1/2) are involved in the cardioprotective effect of adaptation to hypoxia. The role of atrial natriuretic peptide (ANP), erythropoietin, endothelin-1, phosphoinositide 3-kinases (PI3K), protein kinase G (PKG), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38 MAPK) in the protective effect of adaptation to hypoxia requires further research.
Similar content being viewed by others
REFERENCES
Tsibulnikov SY, Maslov LN, Naryzhnaya NV, Ma H, Lishmanov YB, Oeltgen PR, Garlid K (2018) Role of protein kinase C, PI3 kinase, tyrosine kinases, NO-synthase, KATP channels and MPT pore in the signaling pathway of the cardioprotective effect of chronic continuous hypoxia. Gen Physiol Biophys 37:537–547. https://doi.org/10.4149/gpb_2018013
Naryzhnaya NV, Mukhamedzyanov AV, Lasukova TV, Maslov LN (2017) On the participation of the autonomic nervous system in the implementation of the antiarrhythmic effect of adaptation to periodic hypobaric hypoxia. Bull Exp Biol Med 167:275–278. (In Russ).
Meerson FZ, Ustinova EE, Manukhina EB (1989) Prevention of cardiac arrhythmias by adaptation to hypoxia: regulatory mechanisms and cardiotropic effect. Biomed Biochim Acta 48:S83–S88.
Maslov LN, Naryzhnaya N V, Prokudina ES, Kolar F, Gorbunov AS, Zhang Y, Wang H, Tsibulnikov SY, Portnichenko AG, Lasukova T V, Lishmanov YB (2015) Preserved cardiac mitochondrial function and reduced ischaemia/reperfusion injury afforded by chronic continuous hypoxia: role of opioid receptors. Clin Exp Pharmacol Physiol 42:496–501. https://doi.org/10.1111/1440-1681.12383
Winkelmayer WC, Hurley MP, Liu J, Brookhart MA (2012) Altitude and the risk of cardiovascular events in incident US dialysis patients. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc—Eur Ren Assoc 27:2411–2417. https://doi.org/10.1093/ndt/gfr681
Mallet RT, Burtscher J, Richalet J-P, Millet GP, Burtscher M (2021) Impact of High Altitude on Cardiovascular Health: Current Perspectives. Vasc Health Risk Manag 17:317–335. https://doi.org/10.2147/VHRM.S294121
Hampl V, Bíbová J, Banasová A, Uhlík J, Miková D, Hnilicková O, Lachmanová V, Herget J (2006) Pulmonary vascular iNOS induction participates in the onset of chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 290:L11–L20. https://doi.org/10.1152/ajplung.00023.2005
Kylhammar D, Rådegran G (2017) The principal pathways involved in the in vivo modulation of hypoxic pulmonary vasoconstriction, pulmonary arterial remodelling and pulmonary hypertension. Acta Physiol (Oxf) 219:728–756. https://doi.org/10.1111/apha.12749
Maston LD, Jones DT, Giermakowska W, Resta TC, Ramiro-Diaz J, Howard TA, Jernigan NL, Herbert L, Maurice AA, Gonzalez Bosc LV (2018) Interleukin-6 trans-signaling contributes to chronic hypoxia-induced pulmonary hypertension. Pulm Circ 8(3):1–11. https://doi.org/10.1177/2045894018780734
Kentera D, Susić D (1980) Dynamics of regression of right ventricular hypertrophy in rats with hypoxic pulmonary hypertension. Respiration 39:272–275. https://doi.org/10.1159/000194227
Neckář J, Ošťádal B, Kolář F (2004) Myocardial infarct size-limiting effect of chronic hypoxia persists for five weeks of normoxic recovery. Physiol Res 53:621–628.
Yellon DM, Downey JM (2003) Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83:1113–1151. https://doi.org/10.1152/physrev.00009.2003
Maslov LN, Lishmanov YuB (2016) Cardioprotective effect of postconditioning of the heart (experimental and clinical aspects). Tomsk State Univer Control Systems and Radioelectronics, Tomsk. (In Russ).
Yuan X, Zhu D, Guo X, Deng Y, Shang J, Liu K, Liu H (2015) Telmisartan attenuates myocardial apoptosis induced by chronic intermittent hypoxia in rats: modulation of nitric oxide metabolism and inflammatory mediators. Sleep Breath 19:703–709. https://doi.org/10.1007/s11325-014-1081-y
Mallet RT, Manukhina EB, Ruelas SS, Caffrey JL, Downey HF (2018) Cardioprotection by intermittent hypoxia conditioning: evidence, mechanisms, and therapeutic potential. Am J Physiol Heart Circ Physiol 315:H216–H232. https://doi.org/10.1152/ajpheart.00060.2018
Alánová P, Chytilová A, Neckář J, Hrdlička J, Míčová P, Holzerová K, Hlaváčková M, Macháčková K, Papoušek F, Vašinová J, Benák D, Nováková O, Kolář F (2017) Myocardial ischemic tolerance in rats subjected to endurance exercise training during adaptation to chronic hypoxia. J Appl Physiol 122:1452–1461. https://doi.org/10.1152/japplphysiol.00671.2016
La Padula PH, Etchegoyen M, Czerniczyniec A, Piotrkowski B, Arnaiz SL, Milei J, Costa LE (2018) Cardioprotection after acute exposure to simulated high altitude in rats. Role of nitric oxide. Nitric Oxide Biol Chem 73:52–59. https://doi.org/10.1016/j.niox.2017.12.007
La Padula P, Bustamante J, Czerniczyniec A, Costa LE (2008) Time course of regression of the protection conferred by simulated high altitude to rat myocardium: correlation with mtNOS. J Appl Physiol 105:951–957. https://doi.org/10.1152/japplphysiol.90400.2008
Jung F, Palmer LA, Zhou N, Johns RA (2000) Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circ Res 86:319–325. https://doi.org/10.1161/01.res.86.3.319
Yu X, Ge L, Niu L, Lian X, Ma H, Pang L (2018) The Dual Role of Inducible Nitric Oxide Synthase in Myocardial Ischemia/Reperfusion Injury: Friend or Foe? Oxid Med Cell Longev 2018:8364848. https://doi.org/10.1155/2018/8364848
Baker JE, Holman P, Kalyanaraman B, Griffith OW, Pritchard KAJ (1999) Adaptation to chronic hypoxia confers tolerance to subsequent myocardial ischemia by increased nitric oxide production. Ann N Y Acad Sci 874:236–253. https://doi.org/10.1111/j.1749-6632.1999.tb09239.x
Rouet-Benzineb P, Eddahibi S, Raffestin B, Laplace M, Depond S, Adnot S, Crozatier B (1999) Induction of cardiac nitric oxide synthase 2 in rats exposed to chronic hypoxia. J Mol Cell Cardiol 31:1697–1708. https://doi.org/10.1006/jmcc.1999.1005
Nydegger C, Corno AF, von Segesser LK, Beghetti M, Samaja M, Milano G (2019) Effects of PDE-5 Inhibition on the Cardiopulmonary System After 2 or 4 Weeks of Chronic Hypoxia. Cardiovasc Drugs Ther 33:407–414. https://doi.org/10.1007/s10557-019-06887-9
Thompson L, Dong Y, Evans L (2009) Chronic hypoxia increases inducible NOS-derived nitric oxide in fetal guinea pig hearts. Pediatr Res 65:188–192. https://doi.org/10.1203/PDR.0b013e31818d6ad0
Fitzpatrick CM, Shi Y, Hutchins WC, Su J, Gross GJ, Ostadal B, Tweddell JS, Baker JE (2005) Cardioprotection in chronically hypoxic rabbits persists on exposure to normoxia: role of NOS and KATP channels. Am J Physiol Heart Circ Physiol 288:H62–H68. https://doi.org/10.1152/ajpheart.00701.2004
Krylatov AV, Tsibulnikov SY, Mukhomedzyanov AV, Boshchenko AA, Goldberg VE, Jaggi AS, Erben RG, Maslov LN (2021) The Role of Natriuretic Peptides in the Regulation of Cardiac Tolerance to Ischemia/Reperfusion and Postinfarction Heart Remodeling. J Cardiovasc Pharmacol Ther 26:131–148. https://doi.org/10.1177/1074248420952243
Forte M, Madonna M, Schiavon S, Valenti V, Versaci F, Zoccai GB, Frati G, Sciarretta S (2019) Cardiovascular Pleiotropic Effects of Natriuretic Peptides. Int J Mol Sci 20(16): 3874. https://doi.org/10.3390/ijms20163874
Casserly B, Pietras L, Schuyler J, Wang R, Hill NS, Klinger JR (2010) Cardiac atria are the primary source of ANP release in hypoxia-adapted rats. Life Sci 87:382–389. https://doi.org/10.1016/j.lfs.2010.07.013
Lordick F, Hauck RW, Senekowitsch R, Emslander HP (1995) Atrial natriuretic peptide in acute hypoxia-exposed healthy subjects and in hypoxaemic patients. Eur Respir J 8:216–221. https://doi.org/10.1183/09031936.95.08020216
Winter RJ, Meleagros L, Pervez S, Jamal H, Krausz T, Polak JM, Bloom SR (1989) Atrial natriuretic peptide levels in plasma and in cardiac tissues after chronic hypoxia in rats. Clin Sci (Lond) 76:95–101. https://doi.org/10.1042/cs0760095
Bullard AJ, Govewalla P, Yellon DM (2005) Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol 100:397–403. https://doi.org/10.1007/s00395-005-0537-4
Kiss K, Csonka C, Pálóczi J, Pipis J, Görbe A, Kocsis GF, Murlasits Z, Sárközy M, Szűcs G, Holmes CP, Pan Y, Bhandari A, Csont T, Shamloo M, Woodburn KW, Ferdinandy P, Bencsik P (2016) Novel, selective EPO receptor ligands lacking erythropoietic activity reduce infarct size in acute myocardial infarction in rats. Pharmacol Res 113:62–70. https://doi.org/10.1016/j.phrs.2016.08.013
Diab AA, Abulfadle KA, Mohammed NA, Hashim FN (2022) Cardiac and Renal Protective Role of Erythropoietin in a Rat Model of Acute Myocardial Infarction. Zagazig Univ Med J 28:35–44. https://doi.org/10.21608/zumj.2021.55172.2066
Wang S, Azarfar A, Wang Y, Cao Z, Shi Q, Li S (2018) WITHDRAWN:Hematological and vasodilator characteristics for high altitude acclimatization in Holstein heifers ascended to high altitude. Asian-Australasian J Anim Sci 2018:1. https://doi.org/10.5713/ajas.18.0224
Feizi H, Rajaee K, Keyhanmanesh R, Aliparasti MR, Almasi S, Alipour MR (2014) Effect of ghrelin on renal erythropoietin production in chronic hypoxic rats. Endocr Regul 48:3–8. https://doi.org/10.4149/endo_2014_01_3
Schmidt W, Spielvogel H, Eckardt KU, Quintela A, Peñaloza R (1993) Effects of chronic hypoxia and exercise on plasma erythropoietin in high-altitude residents. J Appl Physiol 74:1874–1878. https://doi.org/10.1152/jappl.1993.74.4.1874
Wang P, Gallagher KP, Downey JM, Cohen MV (1996) Pretreatment with endothelin-1 mimics ischemic preconditioning against infarction in isolated rabbit heart. J Mol Cell Cardiol 28:579–588. https://doi.org/10.1006/jmcc.1996.0054
Bugge E, Ytrehus K (1996) Endothelin-1 can reduce infarct size through protein kinase C and KATP channels in the isolated rat heart. Cardiovasc Res 32:920–929
Duda M, Konior A, Klemenska E, Beresewicz A (2007) Preconditioning protects endothelium by preventing ET-1-induced activation of NADPH oxidase and xanthine oxidase in post-ischemic heart. J Mol Cell Cardiol 42:400–410. https://doi.org/10.1016/j.yjmcc.2006.10.014
Blumberg FC, Wolf K, Arzt M, Lorenz C, Riegger GAJ, Pfeifer M (2003) Effects of ET-A receptor blockade on eNOS gene expression in chronic hypoxic rat lungs. J Appl Physiol 94:446–452. https://doi.org/10.1152/japplphysiol.00239.2002
Zhu J, Kang J, Li X, Wang M, Shang M, Luo Y, Xiong M, Hu K (2020) Chronic intermittent hypoxia vs chronic continuous hypoxia: Effects on vascular endothelial function and myocardial contractility. Clin Hemorheol Microcirc 74:417–427. https://doi.org/10.3233/CH-190706
Wang N, Chang Y, Chen L, Guo Y-J, Zhao Y-S, Guo Q-H, Ji E-S (2017) Tanshinone IIA protects against chronic intermittent hypoxia-induced myocardial injury via activating the endothelin 1 pathway. Biomed Pharmacother 95:1013–1020. https://doi.org/10.1016/j.biopha.2017.08.036
Krylatov AV, Maslov LN, Voronkov NS, Boshchenko AA, Popov S V, Gomez L, Wang H, Jaggi AS, Downey JM (2018) Reactive Oxygen Species as Intracellular Signaling Molecules in the Cardiovascular System. Curr Cardiol Rev 14:290–300. https://doi.org/10.2174/1573403X14666180702152436
Lien C-F, Lee W-S, Wang I-C, Chen T-I, Chen T-L, Yang K-T (2018) Intermittent hypoxia-generated ROS contributes to intracellular zinc regulation that limits ischemia/reperfusion injury in adult rat cardiomyocyte. J Mol Cell Cardiol 118:122–132. https://doi.org/10.1016/j.yjmcc.2018.03.014
Chang J-C, Lien C-F, Lee W-S, Chang H-R, Hsu Y-C, Luo Y-P, Jeng J-R, Hsieh J-C, Yang K-T (2019) Intermittent Hypoxia Prevents Myocardial Mitochondrial Ca(2+) Overload and Cell Death during Ischemia/Reperfusion: The Role of Reactive Oxygen Species. Cells 8(6): 564. https://doi.org/10.3390/cells8060564
Shi Z-J, Cheng M, Liu Y-C, Fan X-R, Zhang Y, Wei Y (2020) Effect of chronic intermittent hypobaric hypoxia on heart rate variability in conscious rats. Clin Exp Pharmacol Physiol 47:60–66. https://doi.org/10.1111/1440-1681.13170
Mrakic-Sposta S, Gussoni M, Dellanoce C, Marzorati M, Montorsi M, Rasica L, Pratali L, D’Angelo G, Martinelli M, Bastiani L, Di Natale L, Vezzoli A (2021) Effects of acute and sub-acute hypobaric hypoxia on oxidative stress: a field study in the Alps. Eur J Appl Physiol 121:297–306. https://doi.org/10.1007/s00421-020-04527-x
Balková P, Hlaváčková M, Milerová M, Neckář J, Kolář F, Novák F, Nováková O (2011) N-acetylcysteine treatment prevents the up-regulation of MnSOD in chronically hypoxic rat hearts. Physiol Res 60:467–474. https://doi.org/10.33549/physiolres.932042
Kolár F, Jezková J, Balková P, Breh J, Neckár J, Novák F, Nováková O, Tomásová H, Srbová M, Ost’ádal B, Wilhelm J, Herget J (2007) Role of oxidative stress in PKC-delta upregulation and cardioprotection induced by chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 292:H224-H230. https://doi.org/10.1152/ajpheart.00689.2006
Heusch G (2015) Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116:674–699. https://doi.org/10.1161/CIRCRESAHA.116.305348
Heusch G (2020) Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 17:773–789. https://doi.org/10.1038/s41569-020-0403-y
Morel O-E, Buvry A, Le Corvoisier P, Tual L, Favret F, León-Velarde F, Crozatier B, Richalet J-P (2003) Effects of nifedipine-induced pulmonary vasodilatation on cardiac receptors and protein kinase C isoforms in the chronically hypoxic rat. Pflugers Arch 446:356–364. https://doi.org/10.1007/s00424-003-1034-y
Holzerová K, Hlaváčková M, Žurmanová J, Borchert G, Neckář J, Kolář F, Novák F, Nováková O (2015) Involvement of PKCε in cardioprotection induced by adaptation to chronic continuous hypoxia. Physiol Res 64:191–201. https://doi.org/10.33549/physiolres.932860
Neckár J, Marková I, Novák F, Nováková O, Szárszoi O, Ost’ádal B, Kolár F (2005) Increased expression and altered subcellular distribution of PKC-delta in chronically hypoxic rat myocardium: involvement in cardioprotection. Am J Physiol Heart Circ Physiol 288:H1566-H1572. https://doi.org/10.1152/ajpheart.00586.2004
Naryzhnaya NV, Maslov IN, Khaliulin IG, Zhang Y, Pei JM, Tsepokina AV, Khutornaya MV, Kutikhin AG, Lishmanov YB (2016) Chronic continuous normobaric hypoxia augments cell tolerance to anoxia-reoxygenation: the role of protein kinases. Rus J Physiol 102:1462–1471. (In Russ).
El Alwani M, Usta J, Nemer G, El Sabban M, Nasser M, Bitar H, Souki R, Dbaibo GS, Bitar FF (2005) Regulation of the sphingolipid signaling pathways in the growing and hypoxic rat heart. Prostaglandins Other Lipid Mediat 78:249–263. https://doi.org/10.1016/j.prostaglandins.2005.09.002
Heidbreder M, Naumann A, Tempel K, Dominiak P, Dendorfer A (2008) Remote vs. ischaemic preconditioning: the differential role of mitogen-activated protein kinase pathways. Cardiovasc Res 78:108–115. https://doi.org/10.1093/cvr/cvm114
Ma H-J, Li Q, Ma H-J, Guan Y, Shi M, Yang J, Li D-P, Zhang Y (2014) Chronic intermittent hypobaric hypoxia ameliorates ischemia/reperfusion-induced calcium overload in heart via Na/Ca2+ exchanger in developing rats. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 34:313–324. https://doi.org/10.1159/000363001
Milano G, von Segesser LK, Morel S, Joncic A, Bianciardi P, Vassalli G, Samaja M (2010) Phosphorylation of phosphatidylinositol-3-kinase-protein kinase B and extracellular signal-regulated kinases 1/2 mediate reoxygenation-induced cardioprotection during hypoxia. Exp Biol Med (Maywood) 235:401–410. https://doi.org/10.1258/ebm.2009.009153
Strnisková M, Ravingerová T, Neckár J, Kolár F, Pastoreková S, Barancík M (2006) Changes in the expression and/or activation of regulatory proteins in rat hearts adapted to chronic hypoxia. Gen Physiol Biophys 25:25–41.
Micova P, Hahnova K, Hlavackova M, Elsnicova B, Chytilova A, Holzerova K, Zurmanova J, Neckar J, Kolar F, Novakova O, Novotny J (2016) Chronic intermittent hypoxia affects the cytosolic phospholipase A(2)α/cyclooxygenase 2 pathway via β(2)-adrenoceptor-mediated ERK/p38 stimulation. Mol Cell Biochem 423:151–163. https://doi.org/10.1007/s11010-016-2833-8
Zhang K, Ma Z, Wang W, Liu R, Zhang Y, Yuan M, Li G (2018) Beneficial effects of tolvaptan on atrial remodeling induced by chronic intermittent hypoxia in rats. Cardiovasc Ther 36:e12466. https://doi.org/10.1111/1755-5922.12466
Ling H, Gray CBB, Zambon AC, Grimm M, Gu Y, Dalton N, Purcell NH, Peterson K, Brown JH (2013) Ca2+/Calmodulin-dependent protein kinase II δ mediates myocardial ischemia/reperfusion injury through nuclear factor-κB. Circ Res 112:935–944. https://doi.org/10.1161/CIRCRESAHA.112.276915
Lu H-T, Feng R-Q, Tang J-K, Zhou J-J, Gao F, Ren J (2020) CaMKII/calpain interaction mediates ischemia/reperfusion injury in isolated rat hearts. Cell Death Dis 11:388. https://doi.org/10.1038/s41419-020-2605-y
Zhao P-J, Pan J, Li F, Sun K (2008) Effects of chronic hypoxia on the expression of calmodulin and calcicum/calmodulin-dependent protein kinase II and the calcium activity in myocardial cells in young rats. Zhongguo Dang Dai Er Ke Za Zhi 10:381–385.
Nehra S, Bhardwaj V, Kar S, Saraswat D (2016) Chronic Hypobaric Hypoxia Induces Right Ventricular Hypertrophy and Apoptosis in Rats: Therapeutic Potential of Nanocurcumin in Improving Adaptation. High Alt Med Biol 17:342–352. https://doi.org/10.1089/ham.2016.0032
Xie Y, Zhu W-Z, Zhu Y, Chen L, Zhou Z-N, Yang H-T (2004) Intermittent high altitude hypoxia protects the heart against lethal Ca2+ overload injury. Life Sci 76:559–572. https://doi.org/10.1016/j.lfs.2004.09.017
Gui L, Guo X, Zhang Z, Xu H, Ji Y-W, Wang R-J, Zhu J-H, Chen Q-H (2018) Activation of CaMKIIδA promotes Ca(2+) leak from the sarcoplasmic reticulum in cardiomyocytes of chronic heart failure rats. Acta Pharmacol Sin 39:1604–1612. https://doi.org/10.1038/aps.2018.20
Ravingerová T, Matejíková J, Neckár J, Andelová E, Kolár F (2007) Differential role of PI3K/Akt pathway in the infarct size limitation and antiarrhythmic protection in the rat heart. Mol Cell Biochem 297:111–120. https://doi.org/10.1007/s11010-006-9335-z
Luo G-P, Jian Z, Ma R-Y, Cao Z-Z, Zhu Y, Zhu Y, Tang F-Q, Xiao Y-B (2018) Melatonin alleviates hypoxia-induced cardiac apoptosis through PI3K/Akt pathway. Int J Clin Exp Pathol 11:5840–5849.
García-Niño WR, Zazueta C, Buelna-Chontal M, Silva-Palacios A (2021) Mitochondrial Quality Control in Cardiac-Conditioning Strategies against Ischemia-Reperfusion Injury. Life (Basel, Switzerland) 11(11): 1123. https://doi.org/10.3390/life11111123
Qing M, Görlach A, Schumacher K, Wöltje M, Vazquez-Jimenez JF, Hess J, Seghaye M-C (2007) The hypoxia-inducible factor HIF-1 promotes intramyocardial expression of VEGF in infants with congenital cardiac defects. Basic Res Cardiol 102:224–232. https://doi.org/10.1007/s00395-007-0639-2
Rafiee P, Shi Y, Kong X, Pritchard KAJ, Tweddell JS, Litwin SB, Mussatto K, Jaquiss RD, Su J, Baker JE (2002) Activation of protein kinases in chronically hypoxic infant human and rabbit hearts: role in cardioprotection. Circulation 106:239–245. https://doi.org/10.1161/01.cir.0000022018.68965.6d
Milano G, Morel S, Bonny C, Samaja M, von Segesser LK, Nicod P, Vassalli G (2007) A peptide inhibitor of c-Jun NH2-terminal kinase reduces myocardial ischemia-reperfusion injury and infarct size in vivo. Am J Physiol Heart Circ Physiol 292:H1828-H1835. https://doi.org/10.1152/ajpheart.01117.2006
Shvedova M, Anfinogenova Y, Atochina-Vasserman EN, Schepetkin IA, Atochin DN (2018) c-Jun N-Terminal Kinases (JNKs) in Myocardial and Cerebral Ischemia/Reperfusion Injury. Front Pharmacol 9:715. https://doi.org/10.3389/fphar.2018.00715
Zhao Y-S, An J-R, Yang S, Guan P, Yu F-Y, Li W, Li J-R, Guo Y, Sun Z-M, Ji E-S (2019) Hydrogen and Oxygen Mixture to Improve Cardiac Dysfunction and Myocardial Pathological Changes Induced by Intermittent Hypoxia in Rats. Oxid Med Cell Longev 2019:7415212. https://doi.org/10.1155/2019/7415212
He S, Liu S, Wu X, Xin M, Ding S, Xin D, Ouyang H, Zhang J (2016) Protective role of downregulated MLK3 in myocardial adaptation to chronic hypoxia. J Physiol Biochem 73:371–380. https://doi.org/10.1007/s13105-017-0561-5
Morel S, Milano G, Ludunge KM, Corno AF, Samaja M, Fleury S, Bonny C, Kappenberger L, von Segesser LK, Vassalli G (2006) Brief reoxygenation episodes during chronic hypoxia enhance posthypoxic recovery of LV function: role of mitogen-activated protein kinase signaling pathways. Basic Res Cardiol 101:336–345. https://doi.org/10.1007/s00395-006-0596-1
Li Q, Xiang Y, Chen Y, Tang Y, Zhang Y (2017) Ginsenoside Rg1 Protects Cardiomyocytes Against Hypoxia/Reoxygenation Injury via Activation of Nrf2/HO-1 Signaling and Inhibition of JNK. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 44:21–37. https://doi.org/10.1159/000484578
Wagner C, Tillack D, Simonis G, Strasser RH, Weinbrenner C (2010) Ischemic post-conditioning reduces infarct size of the in vivo rat heart: role of PI3-K, mTOR, GSK-3beta, and apoptosis. Mol Cell Biochem 339:135–147. https://doi.org/10.1007/s11010-009-0377-x
Li W, Zhu L, Ruan Z-B, Wang M-X, Ren Y, Lu W (2019) Nicotinamide protects chronic hypoxic myocardial cells through regulating mTOR pathway and inducing autophagy. Eur Rev Med Pharmacol Sci 23:5503–5511. https://doi.org/10.26355/eurrev_201906_18220
Xie S, Liu W, Jin M, Li X, Wang T, Zeng S, Nie H, Zhao D (2021) Calcineurin suppresses cardiomyocyte-protective autophagy under chronic intermittent hypoxia by downregulating the AMPK pathway. Preprints 2021060605.
Wang J, Maimaitili Y, Zheng H, Yu J, Guo H, Ma H-P, Chen C-L (2017) The influence of rapamycin on the early cardioprotective effect of hypoxic preconditioning on cardiomyocytes. Arch Med Sci 13:947–955. https://doi.org/10.5114/aoms.2016.59712
Cohen MV, Downey JM (2007) Cardioprotection: spotlight on PKG. Br J Pharmacol 152:833–834. https://doi.org/10.1038/sj.bjp.0707453
Xie S, Deng Y, Pan Y-Y, Ren J, Jin M, Wang Y, Wang Z-H, Zhu D, Guo X-L, Yuan X, Shang J, Liu H-G (2016) Chronic intermittent hypoxia induces cardiac hypertrophy by impairing autophagy through the adenosine 5’-monophosphate-activated protein kinase pathway. Arch Biochem Biophys 606:41–52. https://doi.org/10.1016/j.abb.2016.07.006
Gu S, Hua H, Guo X, Jia Z, Zhang Y, Maslov LN, Zhang X, Ma H (2018) PGC-1α Participates in the Protective Effect of Chronic Intermittent Hypobaric Hypoxia on Cardiomyocytes. Cell Physiol Biochem 50:1891–1902. https://doi.org/10.1159/000494869
Zhang H, Liu B, Li T, Zhu Y, Luo G, Jiang Y, Tang F, Jian Z, Xiao Y (2018) AMPK activation serves a critical role in mitochondria quality control via modulating mitophagy in the heart under chronic hypoxia. Int J Mol Med 41:69–76. https://doi.org/10.3892/ijmm.2017.3213
Waskova-Arnostova P, Elsnicova B, Kasparova D, Hornikova D, Kolar F, Novotny J, Zurmanova J (2015) Cardioprotective adaptation of rats to intermittent hypobaric hypoxia is accompanied by the increased association of hexokinase with mitochondria. J Appl Physiol 119:1487–1493. https://doi.org/10.1152/japplphysiol.01035.2014
Waskova-Arnostova P, Kasparova D, Elsnicova B, Novotny J, Neckar J, Kolar F, Zurmanova J (2014) Chronic hypoxia enhances expression and activity of mitochondrial creatine kinase and hexokinase in the rat ventricular myocardium. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 33:310–320. https://doi.org/10.1159/000356671
Nedvedova I, Kolar D, Elsnicova B, Hornikova D, Novotny J, Kalous M, Pravenec M, Neckar J, Kolar F, Zurmanova JM (2018) Mitochondrial genome modulates myocardial Akt/Glut/HK salvage pathway in spontaneously hypertensive rats adapted to chronic hypoxia. Physiol Genomics 50:532–541. https://doi.org/10.1152/physiolgenomics.00040.2017
Kolar D, Gresikova M, Waskova-Arnostova P, Elsnicova B, Kohutova J, Hornikova D, Vebr P, Neckar J, Blahova T, Kasparova D, Novotny J, Kolar F, Novakova O, Zurmanova JM (2017) Adaptation to chronic continuous hypoxia potentiates Akt/HK2 anti-apoptotic pathway during brief myocardial ischemia/reperfusion insult. Mol Cell Biochem 432:99–108. https://doi.org/10.1007/s11010-017-3001-5
Miura T, Miki T (2009) GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ J 73:1184–1192. https://doi.org/10.1253/circj.cj-09-0284
Small BA, Lu Y, Hsu AK, Gross GJ, Gross ER (2015) Morphine Reduces Myocardial Infarct Size via Heat Shock Protein 90 in Rodents. Biomed Res Int 2015:129612. https://doi.org/10.1155/2015/129612
McCarthy J, Lochner A, Opie LH, Sack MN, Essop MF (2011) PKCε promotes cardiac mitochondrial and metabolic adaptation to chronic hypobaric hypoxia by GSK3β inhibition. J Cell Physiol 226:2457–2468. https://doi.org/10.1002/jcp.22592
Gross GJ, Peart JN (2003) KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol 285:H921-H930. https://doi.org/10.1152/ajpheart.00421.2003
Grover GJ, Garlid KD (2000) ATP-sensitive potassium channels: A review of their cardioprotective pharmacology. J Mol Cell Cardiol 32:677–695. https://doi.org/10.1006/jmcc.2000.1111
Peart JN, Gross GJ (2002) Sarcolemmal and mitochondrial K(ATP) channels and myocardial ischemic preconditioning. J Cell Mol Med 6:453–464. https://doi.org/10.1111/j.1582-4934.2002.tb00449.x
Cohen MV, Downey JM (2015) Signalling pathways and mechanisms of protection in pre- and postconditioning: historical perspective and lessons for the future. Br J Pharmacol 172:1913–1932. https://doi.org/10.1111/bph.12903
Crawford RM, Jovanović S, Budas GR, Davies AM, Lad H, Wenger RH, Robertson KA, Roy DJ, Ranki HJ, Jovanović A (2003) Chronic mild hypoxia protects heart-derived H9c2 cells against acute hypoxia/reoxygenation by regulating expression of the SUR2A subunit of the ATP-sensitive K+ channel. J Biol Chem 278:31444–31455. https://doi.org/10.1074/jbc.M303051200
Kolár F, Neckár J, Ostádal B (2005) MCC-134, a blocker of mitochondrial and opener of sarcolemmal ATP-sensitive K+ channels, abrogates cardioprotective effects of chronic hypoxia. Physiol Res 54:467–471.
Naryzhnaya NV, Nackar Ya, Maslov LN, Lishmanov YuB, Kolar F, Lasukova TV (2009) The role of sarcolemmal and mitochondrial KATP-channels in realization of the cardioprotection and antiarrhythmic effect of different regimens of hypobaric adaptation (2009) Rus J Physiol 95: 837–849. (In Russ).
Forkel J, Chen X, Wandinger S, Keser F, Duschin A, Schwanke U, Frede S, Massoudy P, Schulz R, Jakob H, Heusch G (2004) Responses of chronically hypoxic rat hearts to ischemia: KATP channel blockade does not abolish increased RV tolerance to ischemia. Am J Physiol Heart Circ Physiol 286:H545–H551. https://doi.org/10.1152/ajpheart.00022.2003
Funding
This work was supported by the Russian Foundation for Basic Research (RFBR), grant no. 21-515-53003. The role of ERK1/2 in the infarct-limiting effect of chronic hypoxia was evaluated within the state assignment (122020300042-4).
Author information
Authors and Affiliations
Contributions
Conceptualization and design (N.V.N.); reviewing of published data (L.N.M., I.A.D.); writing and editing a manuscript (I.A.D.); selection of information sources (F. Fu).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have neither evident nor potential conflict of interest associated with the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 4, pp. 414–439https://doi.org/10.31857/S0869813922040069.
Rights and permissions
About this article
Cite this article
Naryzhnaya, N.V., Maslov, L.N., Derkachev, I.A. et al. The Significance of NO-Synthase, Reactive Oxygen Species, Kinases and KATP-Channels in the Development of the Infarct-Limiting Effect of Adaptation to Hypoxia. J Evol Biochem Phys 58, 535–547 (2022). https://doi.org/10.1134/S0022093022020211
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022020211