Abstract
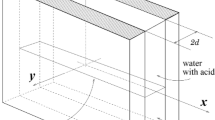
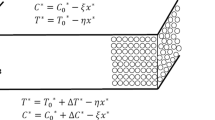
The development of convective instability in a two-layer system of miscible fluids placed in a narrow vertical gap has been studied theoretically and experimentally. The upper and lower layers are formed with aqueous solutions of acid and base, respectively. When the layers are brought into contact, the frontal neutralization reaction begins. We have found experimentally a new type of convective instability, which is characterized by the spatial localization and the periodicity of the structure observed for the first time in the miscible systems. We have tested a number of different acid–base systems and have found a similar patterning there. In our opinion, it may indicate that the discovered effect is of a general nature and should be taken into account in reaction–diffusion–convection problems as another tool with which the reaction can govern the movement of the reacting fluids. We have shown that, at least in one case (aqueous solutions of nitric acid and sodium hydroxide), a new type of instability called as the concentration-dependent diffusion convection is responsible for the onset of the fluid flow. It arises when the diffusion coefficients of species are different and depend on their concentrations. This type of instability can be attributed to a variety of double-diffusion convection. A mathematical model of the new phenomenon has been developed using the system of reaction–diffusion–convection equations written in the Hele–Shaw approximation. It is shown that the instability can be reproduced in the numerical experiment if only one takes into account the concentration dependence of the diffusion coefficients of the reagents. The dynamics of the base state, its linear stability and nonlinear development of the instability are presented. It is also shown that by varying the concentration of acid in the upper layer one can achieve the occurrence of chemo-convective solitary cell in the bulk of an almost immobile fluid. Good agreement between the experimental data and the results of numerical simulations is observed.
Similar content being viewed by others
References
Quincke, G., Über periodische Ausbreitung an Flüssigkeitsoberflächen und dadurch hervorgerufene Bewegungserscheinungen, Ann. Phys., 1888, vol. 271, no. 12, pp. 580–642.
Dupeyrat, M. and Nakache, E., Direct conversion of chemical energy into mechanical energy at an oil water interface, Bioelectroch. Bioener., 1978, vol. 5, no. 1, pp. 134–141.
Kolesnikov, A.K., Thermal explosion in a layer with boundaries at different temperatures in the case of transverse reagent motion, Fiz. Goreniya Vzryva, 1984, vol. 20, no. 3, pp. 64–65.
Thomson, P.J., Batey, W., and Watson, R.J., Interfacial activity in the two phase systems UO2(NO3)2/Pu(NO3)4/HNO3-H2O-TBP/OK, in Proceedings of the Extraction’84, Symposium on Liquid–Liquid Extraction Science, Scotland, Dounreay, November 27–29, 1984, vol. 88, pp. 231–244.
Eckert, K. and Grahn, A., Plume and finger regimes driven by an exothermic interfacial reaction, Phys. Rev. Lett., 1999, vol. 82, no. 22, pp. 4436–4439.
Bratsun, D.A. and de Wit, A., Control of chemoconvective structures in a slab reactor, Tech. Phys., 2008, vol. 53, no. 2, pp. 146–153.
Bratsun, D.A. and de Wit, A., Buoyancy-driven pattern formation in reactive immiscible two-layer systems, Chem. Eng. Sci., 2011, vol. 66, no. 22, pp. 5723–5734.
Eckert, K., Acker, M., and Shi, Y., Chemical pattern formation driven by a neutralization reaction. Mechanism and basic features, Phys. Fluids, 2004, vol. 16, no. 2, pp. 385–399.
Bratsun, D.A., On Rayleigh–Bénard mechanism of alignment of salt fingers in reactive immiscible two-layer systems, Micrograv. Sci. Tech., 2014, vol. 26, no. 5, 293–303.
Shi, Y. and Eckert, K., Orientation-dependent hydrodynamic instabilities from chemo-Marangoni cells to large scale interfacial deformations, Chin. J. Chem. Eng., 2007, vol. 15, no. 5, pp. 748–753.
Bratsun, D.A. and de Wit, A., On Marangoni convective patterns driven by an exothermic chemical reaction in two-layer systems, Phys. Fluids, 2004, vol. 16, no. 4, pp. 1082–1096.
Karlov, S.P., Kazenin, D.A., and Vyazmin, A.V., The time evolution of chemo-gravitational convection on a brim meniscus of wetting, Physica A, 2002, vol. 315, nos. 1–2, pp. 236–242.
Wylock, C., Rednikov, A., Haut, B., and Colinet, P., Nonmonotonic Rayleigh–Taylor instabilities driven by gas-liquid CO2 chemisorption, J. Phys. Chem. B, 2014, vol. 118, no. 38, pp. 11323–11329.
Aitova, E.V. and Bratsun, D.A., Exact solution of chemoconvective stability problem of two-phase liquid-gas system in presence of adsorbed reagent, Vestn. PNRPU, Mekh., 2013, no. 4, pp. 5–17.
Turner, J.S., Double-diffusive phenomena, Ann. Rev. Fluid Mech., 1974, vol. 6, pp. 37–54.
Trevelyan, P.M.J., Almarcha, C., and deWit, A., Buoyancy-driven instabilities around miscible A+B?C reaction fronts: a general classification, Phys. Rev. E, 2015, vol. 91, no. 2, p. 023001.
Almarcha, C., Trevelyan, P.M.J., Grosfils, P., and de Wit, A., Chemically driven hydrodynamic instabilities, Phys. Rev. Lett., 2010, vol. 104, no. 4, p. 044501.
Almarcha, C., R’Honi, Y., de Decker, Y., Trevelyan, P.M.J., Eckert, K., and de Wit, A., Convective mixing induced by acid-base reactions, J. Phys. Chem. B, 2011, vol. 115, no. 32, pp. 9739–9744.
Carballido-Landeira, J., Trevelyan, P.M.J., Almarcha, C., and de Wit, A., Mixed-mode instability of a miscible interface due to coupling between Rayleigh–Taylor and double-diffusive convective modes, Phys. Fluids, 2013, vol. 25, no. 2, p. 024107.
Ash, R. and Espenhahn, S.E., Transport through a slab membrane governed by a concentration-dependent diffusion coefficient. Numerical solution of the diffusion equation: “early-time” and “vt” procedures, J. Membr. Sci., 2000, vol. 180, no. 1, pp. 133–146.
Bowen, W.R. and Williams, P.M., Prediction of the rate of cross-flow ultrafiltration of colloids with concentration-dependent diffusion coefficient and viscosity–theory and experiment, Chem. Eng. Sci., 2001, vol. 56, no. 10, pp. 3083–3099.
Bratsun, D., Kostarev, K., Mizev, A., and Mosheva, E., Concentration-dependent diffusion instability in reactive miscible fluids, Phys. Rev. E, 2015, vol. 92, p. 011003.
Crank, J., The Mathematics of Diffusion, New York: Oxford Univ. Press, 1975.
Chapman, T.W., The transport properties of concentrated electrolytic solutions, PhD (Chem. Eng.) Dissertation, Berkeley: Univ. of California, 1967.
Yeh, H.-S. and Wills, G.B., Diffusion coefficient of aqueous nitric acid at 25° as function of concentration from 0.1 to 1.0 M, J. Chem. Eng. Data, 1971, vol. 16, no. 1, pp. 76–77.
Nisancioglu, K. and Newman, J., Diffusion in aqueous nitric acid solutions, AIChE J., 1973, vol. 19, no. 4, pp. 797–801.
Fary, A.D., The diffusional properties of sodium hydroxide, Doctoral (Phys. Chem.) Dissertation, Appleton, Wisconsin: Inst. Paper Chem., 1966.
Noulty, R.A. and Leaist, D.G., Activity coefficients and diffusion coefficients of dilute aqueous solutions of lithium, sodium, and potassium hydroxides, J. Solution Chem., 1984, vol. 13, no. 11, pp. 767–778.
Harned, H.S. and Shropshire, J.A., The diffusion and activity coefficient of sodium nitrate in dilute aqueous solutions at 25°, J. Am. Chem. Soc., 1958, vol. 80, no. 11, pp. 2618–2619.
Yeh, H.-S. and Wills, G.B., Diffusion coefficient of sodium nitrate in aqueous solution at 25° as a function of concentration from 0.1 to 1.0 M, J. Chem. Eng. Data, 1970, vol. 15, no. 1, pp. 187–189.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Aitova, D.A. Bratsun, K.G. Kostarev, A.I. Mizev, E.A. Mosheva, 2015, published in Vychislitel’naya Mekhanika Sploshnykh Sred, 2015, Vol. 8, No. 4, pp. 345–358.
Rights and permissions
About this article
Cite this article
Aitova, E.V., Bratsun, D.A., Kostarev, K.G. et al. Convective instability in a two-layer system of reacting fluids with concentration-dependent diffusion. J Appl Mech Tech Phy 57, 1226–1238 (2016). https://doi.org/10.1134/S0021894416070026
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0021894416070026