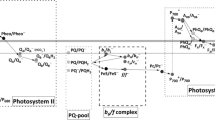
Abstract
This review analyzes data available in the literature on the rates, characteristics, and mechanisms of oxygen reduction to a superoxide anion radical at the sites of photosynthetic electron transport chain where this reduction has been established. The existing assumptions about the role of the components of these sites in this process are critically examined using thermodynamic approaches and results of the recent studies. The process of O2 reduction at the acceptor side of PSI, which is considered the main site of this process taking place in the photosynthetic chain, is described in detail. Evolution of photosynthetic apparatus in the context of controlling the leakage of electrons to O2 is explored. The reasons limiting application of the results obtained with the isolated segments of the photosynthetic chain to estimate the rates of O2 reduction at the corresponding sites in the intact thylakoid membrane are discussed.
Similar content being viewed by others
Abbreviations
- Fd:
-
ferredoxin
- FNR:
-
ferredoxin:NADP+ oxidoreductase
- DCPIP:
-
2,6-dichlorophenolindophenol
- DNP-INT:
-
dinitrophenyl ether 2-iodine-4-nitrotimol
- Em :
-
midpoint redox potential
- PETC:
-
photosynthetic electron transport chain
- PhQ:
-
phylloquinone
- PQ:
-
plastoquinone
- PSI:
-
photosystem I
- PSII:
-
photosystem II
- QA and QB :
-
primary and secondary quinone acceptors of photosystem II, respectively
- QO and QR :
-
quinol-oxidizing (QO site) and quinol-reducing (QR site) sites of the b6f complex, respectively
- ROS:
-
reactive oxygen species
References
Mehler, A. H. (1951) Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents, Arch. Biochem. Biophys., 33, 65-77, https://doi.org/10.1016/0003-9861(51)90082-3.
Ivanov, B. N., Khorobrykh, S. A., Kozuleva, M. A., and Borisova-Mubarakshina, M. M. (2013) The role of oxygen and its reactive species in photosynthesis, in Photosynthesis: Questions to Answer and What We Know Today (Allakhverdiev, S. I., Rubin, A. B., and Shuvalov, V. A., eds) [in Russian], Institute of Computer-Aided Studies, Izhevsk, Vol. 1, pp. 407-460.
Mubarakshina, M. M., and Ivanov, B. N. (2010) The production and scavenging of reactive oxygen species in the plastoquinone pool of chloroplast thylakoid membranes, Physiol. Plant., 140, 103-110, https://doi.org/10.1111/j.1399-3054.2010.01391.x.
Pospíšil, P. (2012) Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II, Biochim. Biophys. Acta, 1817, 218-231, https://doi.org/10.1016/j.bbabio.2011.05.017.
Kozuleva, M. A., and Ivanov, B. N. (2016) The mechanisms of oxygen reduction in the terminal reducing segment of the chloroplast photosynthetic electron transport chain, Plant Cell Physiol., 57, 1397-1404, https://doi.org/10.1093/pcp/pcw035.
Kozuleva, M. A., Ivanov, B. N., Vetoshkina, D. V., and Borisova-Mubarakshina, M. M. (2020) Minimizing an electron flow to molecular oxygen in photosynthetic electron transfer chain: an evolutionary view, Front. Plant Sci., 11, 211, https://doi.org/10.3389/fpls.2020.00211.
Sarewicz, M., Pintscher, S., Pietras, R., Borek, A., Bujnowicz, Ł., Hanke, G., Cramer, W. A., Finazzi, G., and Osyczka, A. (2021) Catalytic reactions and energy conservation in the cytochrome bc1 and b6f complexes of energy-transducing membranes, Chem. Rev., 121, 2020-2108, https://doi.org/10.1021/acs.chemrev.0c00712.
Allen, J. F., and Hall, D. O. (1973) Superoxide reduction as a mechanism of ascorbate-stimulated oxygen uptake by isolated chloroplasts, Biochem. Biophys. Res. Commun., 52, 856-862, https://doi.org/10.1016/0006-291X(73)91016-4.
Asada, K., Kiso, K., and Yoshikawa, K. (1974) Univalent reduction of molecular oxygen by spinach chloroplasts on illumination, J. Biol. Chem., 249, 2175-2181, https://doi.org/10.1016/S0021-9258(19)42815-9.
Wardman, P. (1990) Bioreductive activation of quinones: redox properties and thiol reactivity, Free Radic. Res. Commun., 8, 219-229, https://doi.org/10.3109/10715769009053355.
Takahashi, M., and Asada, K. (1988) Superoxide production in aprotic interior of chloroplast thylakoids, Arch. Biochem. Biophys., 267, 714-722, https://doi.org/10.1016/0003-9861(88)90080-X.
Kozuleva, M., Klenina, I., Proskuryakov, I., Kirilyuk, I., and Ivanov, B. (2011) Production of superoxide in chloroplast thylakoid membranes: ESR study with cyclic hydroxylamines of different lipophilicity, FEBS Lett., 585, 1067-1071, https://doi.org/10.1016/j.febslet.2011.03.004.
Kozuleva, M., Klenina, I., Mysin, I., Kirilyuk, I., Opanasenko, V., Proskuryakov, I., and Ivanov, B. (2015) Quantification of superoxide radical production in thylakoid membrane using cyclic hydroxylamines, Free Radic. Biol. Med., 89, 1014-1023, https://doi.org/10.1016/j.freeradbiomed.2015.08.016.
Kozuleva, M., Goss, T., Twachtmann, M., Rudi, K., Trapka, J., Selinski, J., Ivanov, B., Garapati, P., Steinhoff, H. J., Hase, T., Scheibe, R., Klare, J. P., and Hanke, G. T. (2016) Ferredoxin:NADP(H) oxidoreductase abundance and location influences redox poise and stress tolerance, Plant Physiol., 172, 1480-1493, https://doi.org/10.1104/pp.16.01084.
Fantuzzi, A., Allgöwer, F., Baker, H., McGuire, G., Teh, W. K., Gamiz-Hernandez, A. P., Kaila, V. R. I., and Rutherford, A. W. (2022) Bicarbonate-controlled reduction of oxygen by the QA semiquinone in Photosystem II in membranes, Proc. Natl. Acad. Sci. USA, 119, e2116063119, https://doi.org/10.1073/pnas.2116063119.
Khorobrykh, S. A., and Ivanov, B. N. (2002) Oxygen reduction in a plastoquinone pool of isolated pea thylakoids, Photosynth. Res., 71, 209-219, https://doi.org/10.1023/A:1015583502345.
Ford, R. C., and Evans, M. C. W. (1983) Isolation of a photosystem 2 preparation from higher plants with highly enriched oxygen evolution activity, FEBS Lett., 160, 159-164, https://doi.org/10.1016/0014-5793(83)80957-0.
Fan, D.-Y., Hope, A. B., Smith, P. J., Jia, H., Pace, R. J., Anderson, J. M., and Chow, W. S. (2007) The stoichiometry of the two photosystems in higher plants revisited, Biochim. Biophys. Acta Bioenerg., 1767, 1064-1072, https://doi.org/10.1016/j.bbabio.2007.06.001.
Baniulis, D., Hasan, S. S., Stofleth, J. T., and Cramer, W. A. (2013) Mechanism of enhanced superoxide production in the cytochrome b6f complex of oxygenic photosynthesis, Biochemistry, 52, 8975-8983, https://doi.org/10.1021/bi4013534.
Kozuleva, M., Petrova, A., Milrad, Y., Semenov, A., Ivanov, B., Redding, K. E., and Iftach, Y. (2021) Phylloquinone is the principal Mehler reaction site within photosystem I in high light, Plant Physiol., 186, 1848-1858, https://doi.org/10.1093/plphys/kiab221.
Hosein, B., and Palmer, G. (1983) The kinetics and mechanism of oxidation of reduced spinach ferredoxin by molecular oxygen and its reduced products, Biochim. Biophys. Acta Bioenerg., 723, 383-390, https://doi.org/10.1016/0005-2728(83)90045-2.
Golbeck, J., and Radmer, R. (1984) Is the rate of oxygen uptake by reduced ferredoxin sufficient to account for photosystem I-mediated O2 reduction, Adv. Photosynth. Res., 1, 561.
Böhme, H. (1978) Quantitative determination of ferredoxin, ferredoxin-NADP+ reductase and plastocyanin in spinach chloroplasts, Eur. J. Biochem., 83, 137-141, https://doi.org/10.1111/j.1432-1033.1978.tb12077.x.
McKenzie, S. D., Ibrahim, I. M., Aryal, U. K., and Puthiyaveetil, S. (2020) Stoichiometry of protein complexes in plant photosynthetic membranes, Biochim. Biophys. Acta Bioenerg., 1861, 148141, https://doi.org/10.1016/j.bbabio.2019.148141.
Frankel, L. K., Sallans, L., Limbach, P. A., and Bricker, T. M. (2013) Oxidized amino acid residues in the vicinity of QA and PheoD1 of the photosystem II reaction center: putative generation sites of reducing-side reactive oxygen species, PLoS One, 8, e58042, https://doi.org/10.1371/journal.pone.0058042.
Kale, R., Hebert, A. E., Frankel, L. K., Sallans, L., Bricker, T. M., and Pospíšil, P. (2017) Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of Photosystem II, Proc. Natl. Acad. Sci. USA, 114, 2988-2993, https://doi.org/10.1073/pnas.1618922114.
Kumar, A., Prasad, A., Sedlářová, M., Kale, R., Frankel, L. K., Sallans, L., Bricker, T. M., and Pospíšil, P. (2021) Tocopherol controls D1 amino acid oxidation by oxygen radicals in Photosystem II, Proc. Natl. Acad. Sci. USA, 118, e2019246118, https://doi.org/10.1073/pnas.2019246118.
Taylor, R. M., Sallans, L., Frankel, L. K., and Bricker, T. M. (2018) Natively oxidized amino acid residues in the spinach cytochrome b6f complex, Photosynth. Res., 137, 141-151, https://doi.org/10.1007/s11120-018-0485-0.
Ananyev, G., Renger, G., Wacker, U., and Klimov, V. (1994) The photoproduction of superoxide radicals and the superoxide dismutase activity of Photosystem II. The possible involvement of cytochrome b559, Photosynth. Res., 41, 327-338, https://doi.org/10.1007/BF00019410.
Cleland, R. E., and Grace, S. C. (1999) Voltammetric detection of superoxide production by photosystem II, FEBS Lett., 457, 348-352, https://doi.org/10.1016/S0014-5793(99)01067-4.
Brinkert, K., Causmaecker, S. D., Krieger-Liszkay, A., Fantuzzi, A., and Rutherford, A. W. (2016) Bicarbonate-induced redox tuning in Photosystem II for regulation and protection, Proc. Natl. Acad. Sci. USA, 113, 12144-12149, https://doi.org/10.1073/pnas.1608862113.
Linke, K., and Ho, F. M. (2014) Water in photosystem II: structural, functional and mechanistic considerations, Biochim. Biophys. Acta Bioenerg., 1837, 14-32, https://doi.org/10.1016/j.bbabio.2013.08.003.
Causmaecker, S. D., Douglass, J. S., Fantuzzi, A., Nitschke, W., and Rutherford, A. W. (2019) Energetics of the exchangeable quinone, QB, in Photosystem II, Proc. Natl. Acad. Sci. USA, 116, 19458-19463, https://doi.org/10.1073/pnas.1910675116.
Kruk, J., and Strzałka, K. (1999) Dark reoxidation of the plastoquinone-pool is mediated by the low-potential form of cytochrome b-559 in spinach thylakoids, Photosynth. Res., 62, 273-279, https://doi.org/10.1023/A:1006374319191.
Pospíšil, P., Šnyrychová, I., Kruk, J., Strzałka, K., and Nauš, J. (2006) Evidence that cytochrome b559 is involved in superoxide production in photosystem II: effect of synthetic short-chain plastoquinones in a cytochrome b559 tobacco mutant, Biochem. J., 397, 321-327, https://doi.org/10.1042/BJ20060068.
Müh, F., and Zouni, A. (2016) Cytochrome b 559 in Photosystem II, in Adv. Photosynth. Respir. Springer, Dordrecht, 41, 143-175, https://doi.org/10.1007/978-94-017-7481-9_8.
Shuvalov, V. A., Schreiber, U., and Heber, U. (1994) Spectral and thermodynamic properties of the two hemes of the D1D2cytochrome b-559 complex of spinach, FEBS Lett., 337, 226-230, https://doi.org/10.1016/0014-5793(94)80196-7.
Yadav, D. K., Prasad, A., Kruk, J., and Pospíšil, P. (2014) Evidence for the involvement of loosely bound plastosemiquinones in superoxide anion radical production in photosystem II, PLoS One, 9, e115466, https://doi.org/10.1371/journal.pone.0115466.
Khorobrykh, A. (2019) Hydrogen peroxide and superoxide anion radical photoproduction in PSII preparations at various modifications of the water-oxidizing complex, Plants, 8, 329, https://doi.org/10.3390/plants8090329.
Mubarakshina, M., Khorobrykh, S., and Ivanov, B. (2006) Oxygen reduction in chloroplast thylakoids results in production of hydrogen peroxide inside the membrane, Biochim. Biophys. Acta Bioenerg., 1757, 1496-1503, https://doi.org/10.1016/j.bbabio.2006.09.004.
McCauley, S. W., and Melis, A. (1986) Quantitation of plastoquinone photoreduction in spinach chloroplasts, Photosynth. Res., 8, 3-16, https://doi.org/10.1007/BF00028472.
Khorobrykh, S., Mubarakshina, M., and Ivanov, B. (2004) Photosystem I is not solely responsible for oxygen reduction in isolated thylakoids, Biochim. Biophys. Acta Bioenerg., 1657, 164-167, https://doi.org/10.1016/j.bbabio.2004.04.009.
Forquer, I., Covian, R., Bowman, M. K., Trumpower, B. L., and Kramer, D. M. (2006) Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc1 complex, J. Biol. Chem., 281, 38459-38465, https://doi.org/10.1074/jbc.M605119200.
Vetoshkina, D. V., Ivanov, B. N., Khorobrykh, S. A., Proskuryakov, I. I., and Borisova-Mubarakshina, M. M. (2017) Involvement of the chloroplast plastoquinone pool in the Mehler reaction, Physiol. Plant., 161, 45-55, https://doi.org/10.1111/ppl.12560.
Tikhonov, A. N. (2014) The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways, Plant Physiol. Biochem., 81, 163-183, https://doi.org/10.1016/j.plaphy.2013.12.011.
Kramer, D. M., Crofts, A. R. (1994) Re-examination of the properties and function of the b cytochromes of the thylakoid cytochrome bf complex, Biochim. Biophys. Acta Bioenerg., 1184, 193-201, https://doi.org/10.1016/0005-2728(94)90223-2.
Sang, M., Qin, X. C., Wang, W. D., Xie, J., Chen, X. B., Wang, K. B., Zhang, J. P., Li, L. B., Kuang, T. Y. (2011) High-light-induced superoxide anion radical formation in cytochrome b6f complex from spinach as detected by EPR spectroscopy, Photosynthetica, 49, 48-54, https://doi.org/10.1007/s11099-011-0008-0.
Šnyrychová, I., Pospíšil, P., and Nauš, J. (2006) Reaction pathways involved in the production of hydroxyl radicals in thylakoid membrane: EPR spin-trapping study, Photochem. Photobiol. Sci., 5, 472-476, https://doi.org/10.1039/B514394B.
Kozuleva, M. A., Naidov, I. A., Mubarakshina, M. M., and Ivanov, B. N. (2007) Participation of ferredoxin in oxygen reduction by the photosynthetic electron transport chain, Biophysics, 52, 393-397, https://doi.org/10.1134/S0006350907040069.
Badger, M. R. (1985) Photosynthetic oxygen exchange, Annu. Rev. Plant. Physiol., 36, 27-53, https://doi.org/10.1146/annurev.pp.36.060185.000331.
Allen, J. F. (1975) Oxygen reduction and optimum production of ATP in photosynthesis, Nature, 256, 599-600, https://doi.org/10.1038/256599a0.
Furbank, R. T., and Badger, M. R. (1983) Oxygen exchange associated with electron transport and photophosphorylation in spinach thylakoids, Biochim. Biophys. Acta Bioenerg., 723, 400-409, https://doi.org/10.1016/0005-2728(83)90047-6.
Kozuleva, M. A., and Ivanov, B. N. (2010) Evaluation of the participation of ferredoxin in oxygen reduction in the photosynthetic electron transport chain of isolated pea thylakoids, Photosynth. Res., 105, 51-61, https://doi.org/10.1007/s11120-010-9565-5.
Asada, K. (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons, Annu. Rev. Plant. Physiol. Plant. Mol. Biol., 50, 601-639, https://doi.org/10.1146/annurev.arplant.50.1.601.
Asada, K., and Nakano, Y. (1978) Affinity for oxygen in photoreduction of molecular oxygen and scavenging of hydrogen peroxide in spinach chloroplasts, Photochem. Photobiol., 28, 917-920, https://doi.org/10.1111/j.1751-1097.1978.tb07040.x.
Petrova, A., Mamedov, M., Ivanov, B., Semenov, A., and Kozuleva, M. (2018) Effect of artificial redox mediators on the photoinduced oxygen reduction by photosystem I complexes, Photosynth. Res., 137, 421-429, https://doi.org/10.1007/s11120-018-0514-z.
Robinson, J. M. (1988) Does O2 photoreduction occur within chloroplasts in vivo? Physiol. Plant., 72, 666-680, https://doi.org/10.1111/j.1399-3054.1988.tb09181.x.
Miyake, C., Schreiber, U., Hormann, H., Sano, S., and Asada, K. (1998) The FAD-enzyme monodehydroascorbate radical reductase mediates photoproduction of superoxide radicals in spinach thylakoid membranes, Plant Cell Physiol., 39, 821-829, https://doi.org/10.1093/oxfordjournals.pcp.a029440.
Hanke, G. T., Endo, T., Satoh, F., and Hase, T. (2008) Altered photosynthetic electron channelling into cyclic electron flow and nitrite assimilation in a mutant of ferredoxin:NADP(H) reductase, Plant Cell Environ., 31, 1017-1028, https://doi.org/10.1111/j.1365-3040.2008.01814.x.
Kramer, M., Rodriguez-Heredia, M., Saccon, F., Mosebach, L., Twachtmann, M., Krieger-Liszkay, A., Duffy, C., Knell, R. J., Finazzi, G., and Hanke, G. T. (2021) Regulation of photosynthetic electron flow on dark to light transition by ferredoxin:NADP(H) oxidoreductase interactions, ELife, 10, e56088, https://doi.org/10.7554/eLife.56088.
Buchert, F., Mosebach, L., Gäbelein, P., and Hippler, M. (2020) PGR5 is required for efficient Q cycle in the cytochrome b6f complex during cyclic electron flow, Biochem. J., 477, 1631-1650, https://doi.org/10.1042/BCJ20190914.
Malone, L. A., Proctor, M. S., Hitchcock, A., Hunter, C. N., and Johnson, M. P. (2021) Cytochrome b6f – orchestrator of photosynthetic electron transfer, Biochim. Biophys. Acta Bioenerg., 1862, 148380, https://doi.org/10.1016/j.bbabio.2021.148380.
Kozuleva, M. (2022) Recent advances in the understanding of superoxide anion radical formation in the photosynthetic electron transport chain, Acta Physiol. Plant., 44, 92, https://doi.org/10.1007/s11738-022-03428-0.
Hiyama, T., and Ke, B. (1971) A new photosynthetic pigment, “P430”: its possible role as the primary electron acceptor of photosystem I, Proc. Natl. Acad. Sci. USA, 68, 1010-1013, https://doi.org/10.1073/pnas.68.5.1010.
Kozuleva, M. A., Vetoshkina, D. V., Petrova, A. A., Borisova-Mubarakshina, M. M., and Ivanov, B. N. (2015) The study of oxygen reduction in photosystem I of higher plants using electron donors for this photosystem in intact thylakoids, Biochemistry (Moscow) Suppl. Ser. A Membr. Cell Biol., 9, 246-251, https://doi.org/10.1134/S1990747814060026.
Khorobrykh, S., and Tyystjärvi, E. (2018) Plastoquinol generates and scavenges reactive oxygen species in organic solvent: potential relevance for thylakoids, Biochim. Biophys. Acta Bioenerg., 1859, 1119-1131, https://doi.org/10.1016/j.bbabio.2018.07.003.
Takahashi, M., and Asada, K. (1982) Dependence of oxygen affinity for Mehler reaction on photochemical activity of chloroplast thylakoids, Plant Cell Physiol., 23, 1457-1461, https://doi.org/10.1093/oxfordjournals.pcp.a076495.
Kruk, J., Jemioła-Rzemińska, M., Burda, K., Schmid, G. H., and Strzałka, K. (2003) Scavenging of superoxide generated in photosystem I by plastoquinol and other prenyllipids in thylakoid membranes, Biochemistry, 42, 8501-8505, https://doi.org/10.1021/bi034036q.
Kozuleva, M. A., Petrova, A. A., Mamedov, M. D., Semenov, A. Yu., and Ivanov, B. N. (2014) O2 reduction by photosystem I involves phylloquinone under steady-state illumination, FEBS Lett., 588, 4364-4368, https://doi.org/10.1016/j.febslet.2014.10.003.
Semenov, A. Y., Vassiliev, I. R., van der Est, A., Mamedov, M. D., Zybailov, B., Shen, G., Stehlik, D., Diner, B. A., Chitnis, P. R., and Golbeck, J. H. (2000) Recruitment of a foreign quinone into the A1 site of Photosystem I: altered kinetics of electron transfer in phylloquinone biosynthetic pathway mutants studied by time-resolved optical, EPR, and electrometric techniques, J. Biol. Chem., 275, 23429-23438, https://doi.org/10.1074/jbc.M000508200.
Santabarbara, S., Bullock, B., Rappaport, F., and Redding, K. E. (2015) Controlling electron transfer between the two cofactor chains of photosystem I by the redox state of one of their components, Biophys. J., 108, 1537-1547, https://doi.org/10.1016/j.bpj.2015.01.009.
Kale, R., Sallans, L., Frankel, L. K., and Bricker, T. M. (2020) Natively oxidized amino acid residues in the spinach PS I-LHC I supercomplex, Photosynth. Res., 143, 263-273, https://doi.org/10.1007/s11120-019-00698-7.
Milanovsky, G. E., Petrova, A. A., Cherepanov, D. A., and Semenov, A. Yu. (2017) Kinetic modeling of electron transfer reactions in photosystem I complexes of various structures with substituted quinone acceptors, Photosynth. Res., 133, 185-199, https://doi.org/10.1007/s11120-017-0366-y.
Ivanov, B. (2000) The competition between methyl viologen and monodehydroascorbate radical as electron acceptors in spinach thylakoids and intact chloroplasts, Free Radic. Res., 33, 217-227, https://doi.org/10.1080/10715760000301391.
Bukhov, N. G., Govindachary, S., Egorova, E. A., Joly, D., and Carpentier, R. (2003) N,N,N′,N′-tetramethyl-p-phenylenediamine initiates the appearance of a well-resolved I peak in the kinetics of chlorophyll fluorescence rise in isolated thylakoids, Biochim. Biophys. Acta Bioenerg., 1607, 91-96, https://doi.org/10.1016/j.bbabio.2003.09.002.
Trubitsin, B. V., Mamedov, M. D., Semenov, A. Yu., and Tikhonov, A. N. (2014) Interaction of ascorbate with photosystem I, Photosynth. Res., 122, 215-231, https://doi.org/10.1007/s11120-014-0023-7.
Michelet, L., and Krieger-Liszkay, A. (2012) Reactive oxygen intermediates produced by photosynthetic electron transport are enhanced in short-day grown plants, Biochim. Biophys. Acta Bioenerg., 1817, 1306-1313, https://doi.org/10.1016/j.bbabio.2011.11.014.
Krieger-Liszkay, A., Shimakawa, G., and Sétif, P. (2020) Role of the two PsaE isoforms on O2 reduction at photosystem I in Arabidopsis thaliana, Biochim. Biophys. Acta Bioenerg., 1861, 148089, https://doi.org/10.1016/j.bbabio.2019.148089.
Marco, P., Elman, T., and Yacoby, I. (2019) Binding of ferredoxin NADP+ oxidoreductase (FNR) to plant photosystem I, Biochim. Biophys. Acta Bioenerg., 1860, 689-698, https://doi.org/10.1016/j.bbabio.2019.07.007.
Andersen, B., Scheller, H. V., and Møller, B. L. (1992) The PSI-E subunit of photosystem I binds ferredoxin:NADP+ oxidoreductase, FEBS Lett., 311, 169-173, https://doi.org/10.1016/0014-5793(92)81391-X.
Benz, J. P., Stengel, A., Lintala, M., Lee, Y.-H., Weber, A., Philippar, K., Gügel, I. L., Kaieda, S., Ikegami, T., Mulo, P., Soll, J., and Bölter, B. (2009) Arabidopsis Tic62 and ferredoxin-NADP(H) oxidoreductase form light-regulated complexes that are integrated into the chloroplast redox poise, Plant Cell, 21, 3965-3983, https://doi.org/10.1105/tpc.109.069815.
Jurić, S., Hazler-Pilepić, K., Tomašić, A., Lepeduš, H., Jeličić, B., Puthiyaveetil, S., Bionda, T., Vojta, L., Allen, J. F., Schleiff, E., and Fulgosi, H. (2009) Tethering of ferredoxin:NADP+ oxidoreductase to thylakoid membranes is mediated by novel chloroplast protein TROL, Plant J., 60, 783-794, https://doi.org/10.1111/j.1365-313X.2009.03999.x.
Jagannathan, B., Shen, G., and Golbeck, J. H. (2012) The evolution of type I reaction centers: the response to oxygenic photosynthesis, in Functional Genomics and Evolution of Photosynthetic Systems, Springer, Dordrecht, pp. 285-316, https://doi.org/10.1007/978-94-007-1533-2_12.
Rutherford, A. W., Osyczka, A., and Rappaport, F. (2012) Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: Redox tuning to survive life in O2, FEBS Lett., 586, 603-616, https://doi.org/10.1016/j.febslet.2011.12.039.
Pierella Karlusich, J. J., and Carrillo, N. (2017) Evolution of the acceptor side of photosystem I: ferredoxin, flavodoxin, and ferredoxin-NADP+ oxidoreductase, Photosyn. Res., 134, 235-250, https://doi.org/10.1007/s11120-017-0338-2.
Orf, G. S., Gisriel, C., and Redding, K. E. (2018) Evolution of photosynthetic reaction centers: insights from the structure of the heliobacterial reaction center, Photosynth. Res., 138, 11-37, https://doi.org/10.1007/s11120-018-0503-2.
Hanke, G., and Mulo, P. (2013) Plant type ferredoxins and ferredoxin-dependent metabolism, Plant Cell Environ., 36, 1071-1084, https://doi.org/10.1111/pce.12046.
Fischer, N., Sétif, P., and Rochaix, J.-D. (1997) Targeted mutations in the psaC gene of Chlamydomonas reinhardtii: preferential reduction of FB at low temperature is not accompanied by altered electron flow from photosystem I to ferredoxin, Biochemistry, 36, 93-102, https://doi.org/10.1021/bi962244v.
Shinkarev, V. P., Vassiliev, I. R., and Golbeck, J. H. (2000) A kinetic assessment of the sequence of electron transfer from F(X) to F(A) and further to F(B) in photosystem I: the value of the equilibrium constant between F(X) and F(A), Biophys. J., 78, 363-372, https://doi.org/10.1016/S0006-3495(00)76599-4.
Ptushenko, V. V., Cherepanov, D. A., Krishtalik, L. I., and Semenov, A. Y. (2008) Semi-continuum electrostatic calculations of redox potentials in photosystem I, Photosynth. Res., 97, 55-74, https://doi.org/10.1007/s11120-008-9309-y.
Schoepp-Cothenet, B., van Lis, R., Atteia, A., Baymann, F., Capowiez, L., Ducluzeau, A.-L., Duval, S., Brink, F., Russell, M. J., and Nitschke, W. (2013) On the universal core of bioenergetics, Biochim. Biophys. Acta Bioenerg., 1827, 79-93, https://doi.org/10.1016/j.bbabio.2012.09.005.
Massey, V. (1994) Activation of molecular oxygen by flavins and flavoproteins, J. Biol. Chem., 269, 22459-22462, https://doi.org/10.1016/S0021-9258(17)31664-2.
Ceccarelli, E. A., Arakaki, A. K., Cortez, N., and Carrillo, N. (2004) Functional plasticity and catalytic efficiency in plant and bacterial ferredoxin-NADP(H) reductases, Biochim. Biophys. Acta Proteins Proteomics, 1698, 155-165, https://doi.org/10.1016/j.bbapap.2003.12.005.
Carrillo, N., and Ceccarelli, E. A. (2003) Open questions in ferredoxin-NADP+ reductase catalytic mechanism, Eur. J. Biochem., 270, 1900-1915, https://doi.org/10.1046/j.1432-1033.2003.03566.x.
Batie, C. J., and Kamin, H. (1984) Ferredoxin:NADP+ oxidoreductase. Equilibria in binary and ternary complexes with NADP+ and ferredoxin, J. Biol. Chem., 259, 8832-8839, https://doi.org/10.1016/S0021-9258(17)47229-2.
Mulo, P., and Medina, M. (2017) Interaction and electron transfer between ferredoxin-NADP+ oxidoreductase and its partners: structural, functional, and physiological implications, Photosynth. Res., 134, 265-280, https://doi.org/10.1007/s11120-017-0372-0.
Drachev, L. A., Kaurov, B. S., Mamedov, M. D., Mulkidjanian, A. Y., Semenov, A. Yu, Shinkarev, V. P., Skulachev, V. P., and Verkgovsky, M. I. (1989) Flash-induced electrogenic events in the photosynthetic reaction center and bc1 complexes of Rhodobacter sphaeroides chromatophores, Biochim. Biophys. Acta, 973, 189-197, https://doi.org/10.1016/S0005-2728(89)80421-9.
De Vries, S., Berden, J. A., and Slater, E. C. (1980) Properties of a semiquinone anion located in the QH2:cytochrome c oxidoreductase segment of the mitochondrial respiratory chain, FEBS Lett., 122, 143-148, https://doi.org/10.1016/0014-5793(80)80422-4.
Stroebel, D., Choquet, Y., Popot, J.-L., and Picot, D. (2003) An atypical haem in the cytochrome b(6)f complex, Nature, 426, 413-418, https://doi.org/10.1038/nature02155.
Vilyanen, D., Naydov, I., Ivanov, B., Borisova-Mubarakshina, M., and Kozuleva, M. (2022) Inhibition of plastoquinol oxidation at the cytochrome b6f complex by dinitrophenyl ether of iodonitrothymol (DNP-INT) depends on irradiance and H+ uptake by thylakoid membranes, Biochim. Biophys. Acta Bioenerg., 1863, 148506, https://doi.org/10.1016/j.bbabio.2021.148506.
Schoepp-Cothenet, B., Lieutaud, C., Baymann, F., Verméglio, A., Friedrich, T., Kramer, D. M., and Nitschke, W. (2009) Menaquinone as pool quinone in a purple bacterium, Proc. Natl. Acad. Sci. USA, 106, 8549-8554, https://doi.org/10.1073/pnas.0813173106.
Bergdoll, L., ten Brink, F., Nitschke, W., Picot, D., and Baymann, F. (2016) From low- to high-potential bioenergetic chains: thermodynamic constraints of Q-cycle function, Biochim. Biophys. Acta Bioenerg., 1857, 1569-1579, https://doi.org/10.1016/j.bbabio.2016.06.006.
Alric, J., Pierre, Y., Picot, D., Lavergne, J., and Rappaport, F. (2005) Spectral and redox characterization of the heme ci of the cytochrome bf complex, Proc. Natl. Acad. Sci. USA, 102, 15860-15865, https://doi.org/10.1073/pnas.0508102102.
Gisriel, C., Sarrou, I., Ferlez, B., Golbeck, J. H., Redding, K. E., and Fromme, R. (2017) Structure of a symmetric photosynthetic reaction center-photosystem, Science, 357, 1021-1025, https://doi.org/10.1126/science.aan5611.
He, Z., Ferlez, B., Kurashov, V., Tank, M., Golbeck, J. H., and Bryant, D. A. (2019) Reaction centers of the thermophilic microaerophile, Chloracidobacterium thermophilum (Acidobacteria) I: biochemical and biophysical characterization, Photosynth. Res., 142, 87-103, https://doi.org/10.1007/s11120-019-00650-9.
Su, X., Ma, J., Pan, X., Zhao, X., Chang, W., Liu, Z., Zhang, X., and Li, M. (2019) Antenna arrangement and energy transfer pathways of a green algal photosystem-I-LHCI supercomplex, Nat. Plants, 5, 273-281, https://doi.org/10.1038/s41477-019-0380-5.
Kashey, T. S., Luu, D. D., Cowgill, J. C., Baker, P. L., and Redding, K. E. (2018) Light-driven quinone reduction in heliobacterial membranes, Photosynth. Res., 138, 1-9, https://doi.org/10.1007/s11120-018-0496-x.
McConnell, M. D., Cowgill, J. B., Baker, P. L., Rappaport, F., and Redding, K. E. (2011) Double reduction of plastoquinone to plastoquinol in photosystem 1, Biochemistry, 50, 11034-11046, https://doi.org/10.1021/bi201131r.
Guergova-Kuras, M., Boudreaux, B., Joliot, A., Joliot, P., and Redding, K. (2001) Evidence for two active branches for electron transfer in photosystem I, Proc. Natl. Acad. Sci. USA, 98, 4437-4442, https://doi.org/10.1073/pnas.081078898.
Ksas, B., Alric, J., Caffarri, S., and Havaux, M. (2022) Plastoquinone homeostasis in plant acclimation to light intensity, Photosynth. Res., 152, 43-54, https://doi.org/10.1007/s11120-021-00889-1.
Suslichenko, I. S., and Tikhonov, A. N. (2019) Photo-reducible plastoquinone pools in chloroplasts of Tradescentia plants acclimated to high and low light, FEBS Lett., 593, 788-798, https://doi.org/10.1002/1873-3468.13366.
Acknowledgments
The authors would like to thank Dr. M. M. Borisova for valuable discussions during preparation of the review.
Funding
This work was financially supported by the Russian Science Foundation, grant no. 22-24-01074.
Author information
Authors and Affiliations
Contributions
M.A.K. – writing the text; B.N.I. – editing the article.
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain descriptions of any research involving human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kozuleva, M.A., Ivanov, B.N. Superoxide Anion Radical Generation in Photosynthetic Electron Transport Chain. Biochemistry Moscow 88, 1045–1060 (2023). https://doi.org/10.1134/S0006297923080011
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923080011