Abstract
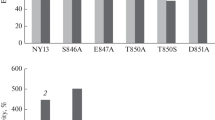
The plasma membrane Pma1 H+-ATPase of the yeast Saccharomyces cerevisiae contains conserved residue Asp739 located at the interface of transmembrane segment M6 and the cytosol. Its replacement by Asn or Val (Petrov et al. (2000) J. Biol. Chem., 275, 15709-15716) or by Ala (Miranda et al. (2011) Biochim. Biophys. Acta, 1808, 1781-1789) caused complete blockage of biogenesis of the enzyme, which did not reach secretory vesicles. It was proposed that a strong ionic bond (salt bridge) could be formed between this residue and positively charged residue(s) in close proximity, and the replacement D739A disrupted this bond. Based on a 3D homology model of the enzyme, it was suggested that the conserved Arg811 located in close proximity to Asp739 could be such stabilizing residue. To test this suggestion, single mutants with substituted Asp739 (D739V, D739N, D739A, and D739R) and Arg811 (R811L, R811M, R811A, and R811D) as well as double mutants carrying charge-neutralizing (D739A/R811A) or charge-swapping (D739R/R811D) substitutions were used. Expression of ATPases with single substitutions R811A and R811D were 38-63%, and their activities were 29-30% of the wild type level; ATP hydrolysis and H+ transport in these enzymes were essentially uncoupled. For the other substitutions including the double mutations, the biogenesis of the enzyme was practically blocked. These data confirm the important role of Asp739 and Arg811 residues for the biogenesis and function of the enzyme, suggesting their importance for defining H+ transport determinants but ruling out, however, the existence of a strong ionic bond (salt bridge) between these two residues and/or importance of such bridge for structure–function relationships in Pma1 H+-ATPase.
Similar content being viewed by others
References
Serrano, R., Kielland-Brandt, M. C., and Fink, G. R. (1986) Yeast plasma membrane ATPase is essential for growth and has homology with (Na+,K+)K+and Ca2+ATPases, Nature, 319, 689–693.
Lutsenko, S., and Kaplan, J. H. (1995) Organization of Ptype ATPases: significance of structural diversity, Biochemistry, 34, 15607–15613.
Axelsen, K. B., and Palmgren, M. G. (1998) Evolution of substrate specificities in the P-type ATPase superfamily, J. Mol. Evol., 46, 84–101.
Petrov, V. V., and Okorokov, L. A. (1992) Energization of yeast plasmalemma is necessary for activation of its ase by glucose, Biokhimiya (Moscow), 57, 1705–1711.
Goffeau, A., and Slayman, C. W. (1981) The protontranslocating ATPase of the fungal plasma membrane, Biochim. Biophys. Acta, 639, 197–223.
Andersen, J. P., and Vilsen, B. (1994) Amino acids Asn796 and Thr799 of the Ca2+-ATPase of sarcoplasmic reticulum bind Ca2+ at different sites, J. Biol. Chem., 269, 15931–15936.
Rice, W. J., and MacLennan, D. H. (1996) Scanning mutagenesis reveals a similar pattern of mutation sensitivity in transmembrane sequences M4, M5, and M6, but not in M8, of the Ca2+-ATPase of sarcoplasmic reticulum (SERCA1a), J. Biol. Chem., 271, 31412–31419.
Zhang, Z., Lewis, D., Strock, C., Inesi, G., Nakasako, M., Nomura, H., and Toyoshima, C. (2000) Detailed characterization of the cooperative mechanism of Ca2+ binding and catalytic activation in the Ca2+ transport (SERCA) ATPase, Biochemistry, 39, 8758–8767.
Vilsen, B., and Andersen, J. P. (1998) Mutation to the glutamate in the fourth membrane segment of Na+,K+ATPase and Ca2+-ATPase affects cation binding from both sides of the membrane and destabilizes the occluded enzyme forms, Biochemistry, 37, 10961–10971.
Jewell-Motz, E. A., and Lingrel, J. B. (1993) Site-directed mutagenesis of the Na,K-ATPase: consequences of substitutions of negatively-charged amino acids localized in the transmembrane domains, Biochemistry, 32, 13523–13530.
Kuntzweiler, T. A., Arguello, J. M., and Lingrel, J. B. (1996) Asp804 and Asp808 in the transmembrane domain of the Na,K-ATPase a subunit are cation coordinating residues, J. Biol. Chem., 271, 29682–29687.
Nielsen, J. M., Pedersen, P. A., Karlish, S. J. D., and Jorgensen, P. L. (1998) Importance of intramembrane carboxylic acids for occlusion of K+ ions at equilibrium in renal Na,K-ATPase, Biochemistry, 37, 1961–1968.
Swarts, H. G., Klaassen, C. H., De Boer, M., Fransen, J. A., and De Pont, J. J. (1996) Role of negatively charged residues in the fifth and sixth transmembrane domains of the catalytic subunit of gastric H+,K+-ATPase, J. Biol. Chem., 271, 29764–29772.
Hermsen, H. P., Koenderink, J. B., Swarts, H. G., and De Pont, J. J. (1998) The negative charge of glutamic acid-795 is essential for gastric H+,K+-ATPase activity, Biochemistry, 39, 1330–1337.
Hermsen, H. P., Swarts, H. G., Koenderink, J. B., and De Pont, J. J. (2000) The carbonyl group of glutamic acid-820 in the gastric H+,K+-ATPase alpha-subunit is essential for K+ activation of the enzyme activity, Biochem. J., 331, 465472.
Swarts, H. G. P., Koenderink, J. B., Willems, P. H., Krieger, E., and De Pont, J. J. (2005) Asn792 participates in the hydrogen bond network around the K+-binding pocket of gastric H,K-ATPase, J. Biol. Chem., 280, 1148811494.
Asano, S., Io, T., Kimura, T., Sakamoto, S., and Takeguchi, N. (2001) Alanine-scanning mutagenesis of the sixth transmembrane segment of gastric H+,K+-ATPase alpha-subunit, J. Biol. Chem., 276, 31265–31273.
Asano, S., Morii, M., and Takeguchi, N. (2004) Molecular and cellular regulation of the gastric pump, Biol. Pharm. Bull., 27, 1–12.
Buch-Pedersen, M. J., Venema, K., Serrano, R., and Palmgren, M. G. (2000) Abolishment of proton pumping and accumulation in the E1P conformational state of a plant plasma membrane H+-ATPase by substitution of a conserved aspartyl residue in transmembrane segment 6, J. Biol. Chem., 275, 39167–39173.
Buch-Pedersen, M. J., and Palmgren, M. G. (2003) Conserved Asp684 in transmembrane segment M6 of the plant plasma membrane P-type proton pump AHA2 is molecular determinant of proton translocation, J. Biol. Chem., 278, 17845–17851.
Wei, Y., Chen, J., Rosas, G., Tompkins, D. A., Holt, P. A., and Rao, R. (2000) Phenotypic screening of mutations in Pmr1, the yeast secretory pathway Ca2+/Mn2+-ATPase, reveals residues critical for ion selectivity and transport, J. Biol. Chem., 275, 23927–23932.
Mandal, D., Woolf, T. B., and Rao, R. (2000) Manganese selectivity of pmr1, the yeast secretory pathway ion pump, is defined by residue Gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport, J. Biol. Chem., 275, 23933–23938.
Ambesi, A., Pan, R. L., and Slayman, C. W. (1996) Alanine-scanning mutagenesis along membrane segment 4 of the yeast plasma membrane H+-ATPase. Effects on structure and function, J. Biol. Chem., 271, 22999–23005.
Dutra, M. B., Ambesi, A., and Slayman, C. W. (1998) Structure-function relationships in membrane segment 5 of the yeast Pma1 H+-ATPase, J. Biol. Chem., 273, 17411–17417.
Petrov, V. V., Padmanabha, K. P., Nakamoto, R. K., Allen, K. E., and Slayman, C. W. (2000) Functional role of charged residues in the transmembrane segments of the yeast plasma membrane H+-ATPase, J. Biol. Chem., 275, 15709–15716.
Guerra, G., Petrov, V. V., Allen, K. E., Miranda, M., Pardo, J. P., and Slayman, C. W. (2007) Role of transmembrane segment M8 in the biogenesis and function of yeast plasma-membrane H+-ATPase, Biochim. Biophys. Acta, 1768, 2383–2392.
Miranda-Arango, M., Pardo, J. P., and Petrov, V. V. (2009) Role of transmembrane segment M6 in the biogenesis and function of the yeast Pma1 H+-ATPase, J. Biomol. Struct. Dyn., 26, 866–868.
Petrov, V. V. (2009) Heat shock affects functioning of the yeast Pma1 H+-ATPase, J. Biomol. Struct. Dyn., 26, 857–858.
Petrov, V. V. (2010) Point mutations in Pma1 H+-ATPase of Saccharomyces cerevisiae: influence on its expression and activity, Biochemistry (Moscow), 75, 1055–1064.
Miranda, M., Pardo, J. P., and Petrov, V. V. (2011) Structure-function relationships in membrane segment 6 of the yeast plasma membrane Pma1 H+-ATPase, Biochim. Biophys. Acta, 1808, 1781–1789.
Petrov, V. V. (2011) Role of M5-M6 loop in the biogenesis and function of the yeast Pma1 H+-ATPase, J. Biomol. Struct. Dyn., 28, 1024–1025.
Petrov, V. V. (2015) Point mutations in the extracytosolic loop between transmembrane segments M5 and M6 of the yeast Pma1 H+-ATPase: alanine-scanning mutagenesis, J. Biomol. Struct. Dyn., 33, 70–84.
Petrov, V. V. (2015) Role of loop L5-6 connecting transmembrane segments M5 and M6 in biogenesis and functioning of yeast Pma1 H+-ATPase, Biochemistry (Moscow), 80, 31–44.
Toyosima, C., Nakasako, M., Nomura, H., and Ogawa, H. (2000) Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution, Nature, 405, 647–655.
Toyosima, C., and Nomura, H. (2002) Structural changes in the calcium pump accompanying the dissociation of calcium, Nature, 418, 605–611.
Takahashi, M., Kondou, Y., and Toyoshima, C. (2007) Interdomain communication in calcium pump as revealed in the crystal structures with transmembrane inhibitors, Proc. Natl. Acad. Sci. USA, 104, 5800–5805.
Toyoshima, C., Norimatsu, Y., Iwasawa, S., Tsuda, T., and Ogawa, H. (2007) How processing of aspartyl phosphate is coupled to lumenal gating of the ion pathway in the calcium pump, Proc. Natl. Acad. Sci. USA, 104, 19831–19836.
Toyoshima, C. (2008) Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum, Arch. Biochem. Biophys., 476, 3–11.
Toyoshima, C., Iwasawa, S., Ogawa, H., Hirata, A., Tsueda, J., and Inesi, G. (2013) Crystal structures of the calcium pump and sarcolipin in the Mg2+-bound E1 state, Nature, 495, 260–264.
Morth, J. P., Pedersen, B. P., Toustrup-Jensen, M. S., Sorensen, T. L., Petersen, J., Andersen, J. P., Vilsen, B., and Nissen, P. (2007) Crystal structure of the sodiumpotassium pump, Nature, 450, 1043–1049.
Shinoda, T., Ogawa, H., Cornelius, F., and Toyosima, C. (2009) Crystal structure of the sodium-potassium pump at 2.4 Å resolution, Nature, 459, 446–450.
Ogawa, H., Shinoda, T., Cornelius, F., and Toyoshima, C. (2009) Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain, Proc. Natl. Acad. Sci. USA, 106, 13742–13747.
Nyblom, M., Poulsen, H., Gourdon, P., Reinhard, L., Andersson, M., Lindahl, E., Fedosova, N., and Nissen, P. (2013) Crystal structure of Na+, K+-ATPase in the Na+bound state, Science, 342, 123–127.
Kanai, R., Ogawa, H., Vilsen, B., Cornelius, F., and Toyoshima, C. (2013) Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state, Nature, 502, 201206.
Pedersen, B. P., Buch-Pedersen, M., Morth, J. J. P., Palmgren, M. G., and Nissen, P. (2007) Crystal structure of the plasma membrane proton pump, Nature, 450, 11111114.
Gupta, S. S., DeWitt, N. D., Allen, K. E., and Slayman, C. W. (1998) Evidence for a salt bridge between transmembrane segments 5 and 6 of the yeast plasma-membrane H+ATPase, J. Biol. Chem., 273, 34328–34334.
Nakamoto, R. K., Rao, R., and Slayman, C. W. (1991) Expression of the yeast plasma membrane H+-ATPase in secretory vesicles. A new strategy for directed mutagenesis, J. Biol. Chem., 266, 7940–7949.
Petrov, V. V., and Slayman, C. W. (1995) Site-directed mutagenesis of the yeast PMA1 H+-ATPase. Structural and functional role of cysteine residues, J. Biol. Chem., 270, 28535–28540.
Fabiato, A., and Fabiato, F. (1979) Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells, J. Physiol. (Paris), 75, 463–505.
Fiske, C. H., and Subbarow, Y. (1925) The colorimetric determination of phosphorus, J. Biol. Chem., 66, 375–400.
Bensadoun, A., and Weinstein, D. (1976) Assay of proteins in the presence of interfering materials, Anal. Biochem., 70, 241–250.
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice, Nucleic Acids Res., 22, 4673–4680.
Serrano, R. (1988) Structure and function of proton translocating ATPase in plasma membranes of plants and fungi, Biochim. Biophys. Acta, 947, 1–28.
Ambesi, A., Miranda, M., Petrov, V. V., and Slayman, C. W. (2000) Biogenesis and function of the yeast plasma-membrane H+-ATPase, J. Exp. Biol., 203, 156–160.
Ferreira, T., Mason, A. B., Pypaert, M., Allen, K. E., and Slayman, C. W. (2002) Quality control in the yeast secretory pathway: a misfolded PMA1 H+-ATPase reveals two checkpoints, J. Biol. Chem., 277, 21027–21040.
Mason, A. B., Allen, K. E., and Slayman, C. W. (2014) Cterminal truncations of the Saccharomyces cerevisiae PMA1 H+-ATPase have major impacts on protein conformation, trafficking, quality control, and function, Eukaryot. Cell, 13, 43–52.
Nakamoto, R. K., Verjovski-Almeida, S., Allen, K. E., Ambesi, A., Rao, R., and Slayman, C. W. (1998) Substitutions of aspartate 378 in the phosphorylation domain of the yeast PMA1 H+-ATPase disrupt protein folding and biogenesis, J. Biol. Chem., 273, 7338–7344.
Dougherty, D. A. (2006) Modern Physical Organic Chemistry, University Science Books, Sausalito, CA.
Bairagya, H. R., Mukhopadhyay, B. P., and Bera, A. K. (2011) Role of salt bridge dynamics in inter domain recognition of human IMPDH isoforms: insight to inhibitor topology for isoform II, J. Biomol. Struct. Dyn., 29, 441–462.
Bairagya, H. R., and Mukhopadhyay, B. P. (2013) An insight to the dynamics of conserved water-mediated salt bridge interaction and interdomain recognition in hIMPDH isoforms, J. Biomol. Struct. Dyn., 31, 788–808.
Morozov, V. N., and Kallenbach, N. R. (1996) Stabilization of helical peptides by mixed spaced salt bridges, J. Biomol. Struct. Dyn., 14, 285–291.
Hendsch, Z. S., and Tidor, B. (1994) Do salt bridges stabilize proteins? A continuum electrostatic analysis, Protein Sci., 3, 211–226.
Sindelar, C. V., Hendsch, Z. S., and Tidor, B. (1998) Effects of salt bridges on protein structure and design, Protein Sci., 7, 1898–1914.
Strop, P., and Mayo, S. L. (2000) Contribution of surface salt bridges to protein stability, Biochemistry, 39, 1251–1255.
Kumar, S., and Nussinov, R. (2002) Close-range electrostatic interactions in proteins, ChemBioChem, 3, 604–617.
Kumar, S., Tsai, C.-J., Ma, B., and Nussinov, R. (2000) Contribution of salt bridges toward protein thermostability, J. Biomol. Struct. Dyn., 17, S1, 79–85.
Panja, A. S., Bandopadhyay, B., and Maiti, S. (2015) Protein thermostability is owing to their preferences to non-polar smaller volume amino acids, variations in residual physico-chemical properties and more salt-bridges, PLoS, doi: 10.1371/journal.pone.0131495.
Frillingos, S., Sahin-Toth, M., Lengeler, J. W., and Kaback, H. R. (1995) Helix packing in the sucrose permease of Escherichia coli: properties of engineered charge pairs between helixes VII and XI, Biochemistry, 34, 9368–9373.
Frillingos, S., and Kaback, H. R. (1995) Chemical rescue of Asp237>Ala and Lys358>Ala mutants in the lactose permease of Escherichia coli, Biochemistry, 35, 13363–13367.
Weinglass, A., Whitelegge, J. P., Faull, K. F., and Kaback, H. R. (2004) Monitoring conformational rearrangements in the substrate-binding site of a membrane transport protein by mass spectrometry, J. Biol. Chem., 279, 4185841865.
Guan, L., and Kaback, H. R. (2009) Properties of a LacY efflux mutant, Biochemistry, 48, 9250–9255.
Koenderink, J. B., Swarts, H. G. P., Willems, P. H., Krieger, E., and De Pont, J. J. (2004) A conformation-specific interhelical salt bridge in the K+ binding site of gastric H,K-ATPase, J. Biol. Chem., 279, 16417–16424.
Durr, K. L., Seuffert, I., and Friedrich, T. (2010) Deceleration of the E1P-E2P transition and ion transport by mutation of potentially salt bridge-forming residues Lys791 and Glu-820 in gastric H+/K+-ATPase, J. Biol. Chem., 285, 39366–39379.
Jorgensen, P. L., Hakansson, K. O., and Karlish, S. J. (2003) Structure and mechanism of Na,K-ATPase: functional sites and their interactions, Annu. Rev. Physiol., 65, 817–849.
Rao, U. S., and Scarborough, G. A. (1990) Chemical state of the cysteine residues in the Neurospora crassa plasma membrane H+-ATPase, J. Biol. Chem., 265, 7227–7235.
Roblez-Martinez, L., Pardo, J. P., Miranda, M., Mendez, T. L., Matus-Ortega, M. G., Mendoza-Hernandez, G., and Guerra-Sanchez, G. (2013) The basidiomycete Ustilago maydis has two plasma membrane H+-ATPases related to fungi and plants, J. Bionerg. Biomembr., 45, 477–290.
Supply, P., Wach, A., Thines-Sempoux, D., and Goffeau, A. (1993) Proliferation of intracellular structures upon overexpression of the PMA2 ATPase in Saccharomyces cerevisiae, J. Biol. Chem., 268, 19744–19752.
Supply, P., Wach, A., and Goffeau, A. (1993) Enzymatic properties of the PMA2 plasma membrane-bound H+ATPase of Saccharomyces cerevisiae, J. Biol. Chem., 268, 19753–19759.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2017, Vol. 82, No. 1, pp. 121-136. Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM16-236, November 7, 2016.
In memory of C. W. Slayman
Rights and permissions
About this article
Cite this article
Petrov, V.V. Functioning of yeast Pma1 H+-ATPase under changing charge: Role of Asp739 and Arg811 residues. Biochemistry Moscow 82, 46–59 (2017). https://doi.org/10.1134/S0006297917010059
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297917010059