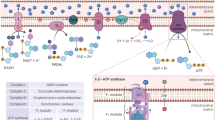
Abstract
The role of mitochondria in oxidative stress is well recognized, but many questions are still to be answered. This article is intended to update our comprehensive review in 2005 by highlighting the progress in understanding of mitochondrial reactive oxygen species (ROS) metabolism over the past 10 years. We review the recently identified or re-appraised sources of ROS generation in mitochondria, such as p66shc protein, succinate dehydrogenase, and recently discovered properties of the mitochondrial antioxidant system. We also reflect upon some controversies, disputes, and misconceptions that confound the field.
Similar content being viewed by others
Abbreviations
- DCF:
-
dichlorofluorescin
- DCF-DA:
-
dichlorofluorescin diacetate
- DHO:
-
dihydroorotate
- DHO DH:
-
dihydroorotate dehydrogenase
- DLD:
-
dihydrolipoamide dehydrogenase
- ETF:
-
electron-transferring flavoprotein
- F.e.T.:
-
forward electron transport
- GPx1:
-
glutathione peroxidase 1
- GR:
-
glutathione reductase
- HRP:
-
horseradish peroxidase
- IDH:
-
isocitrate dehydrogenase, NADP-dependent
- IM:
-
inner mitochondrial membrane
- ME:
-
NADP-dependent malic enzyme
- MnSOD:
-
manganese-containing superoxide dismutase (SOD2)
- Prx3:
-
peroxiredoxins 3
- Prx5:
-
peroxiredoxins 5
- R.e.T.:
-
reverse electron transport
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- TH:
-
transhydrogenase
- Trx2-ox:
-
thioredoxin 2 oxidized
- Trx2-red:
-
thioredoxin 2 reduced
- TrxR2:
-
thioredoxin reductase 2
- TTFA:
-
thenoyltrifluoroacetone
- Δψ:
-
membrane potential
References
Zimorski, V., Ku, C., Martin, W. F., and Gould, S. B. (2014) Endosymbiotic theory for organelle origins, Curr. Opin. Microbiol., 22C, 38–48.
Galluzzi, L., Bravo-San Pedro, J. M., and Kroemer, G. (2014) Organelle-specific initiation of cell death, Nat. Cell Biol., 16, 728–736.
Skulachev, V. P. (2000) Mitochondria in the programmed death phenomena; a principle of biology: “it is better to die than to be wrong”, IUBMB Life, 49, 365–373.
Kushnareva, Y., and Newmeyer, D. D. (2010) Bioenergetics and cell death, Ann. NY Acad. Sci., 1201, 50–57.
Tait, S. W., and Green, D. R. (2012) Mitochondria and cell signaling, J. Cell Sci., 125, 807–815.
Andreyev, A. Y., Kushnareva, Y. E., and Starkov, A. A. (2005) Mitochondrial metabolism of reactive oxygen species, Biochemistry (Moscow), 70, 200–214.
Drose, S., Brandt, U., and Wittig, I. (2014) Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation, Biochim. Biophys. Acta, 1844, 1344–1354.
Grivennikova, V. G., and Vinogradov, A. D. (2013) Mitochondrial production of reactive oxygen species, Biochemistry (Moscow), 78, 1490–1511.
Kaludercic, N., Deshwal, S., and Di Lisa, F. (2014) Reactive oxygen species and redox compartmentalization, Front. Physiol., 5, 285.
Lambert, A. J., and Brand, M. D. (2009) Reactive oxygen species production by mitochondria, Methods Mol. Biol., 554, 165–181.
Murphy, M. P. (2009) How mitochondria produce reactive oxygen species, Biochem. J., 417, 1–13.
Murphy, M. P. (2014) Antioxidants as therapies: can we improve on nature? Free Radic. Biol. Med., 66, 20–23.
Skulachev, V. P. (2013) Cationic antioxidants as a powerful tool against mitochondrial oxidative stress, Biochem. Biophys. Res. Commun., 44, 275–279.
Zorov, D. B., Juhaszova, M., and Sollott, S. J. (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release, Physiol. Rev., 94, 909–950.
Starkov, A. A. (2008) The role of mitochondria in reactive oxygen species metabolism and signaling, Ann. NY Acad. Sci., 1147, 37–52.
Boveris, A., and Chance, B. (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen, Biochem. J., 134, 707–716.
Kareyeva, A. V., Grivennikova, V. G., and Vinogradov, A. D. (2012) Mitochondrial hydrogen peroxide production as determined by the pyridine nucleotide pool and its redox state, Biochim. Biophys. Acta, 1817, 1879–1885.
Kussmaul, L., and Hirst, J. (2006) The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria, Proc. Natl. Acad. Sci. USA, 103, 7607–7612.
Vinogradov, A. D., and Grivennikova, V. G. (2005) Generation of superoxide-radical by the NADH:ubiquinone oxidoreductase of heart mitochondria, Biochemistry (Moscow), 70, 120–127.
Hoffman, D. L., and Brookes, P. S. (2009) Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions, J. Biol. Chem., 284, 16236–16245.
Hoffman, D. L., Salter, J. D., and Brookes, P. S. (2007) Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling, Am. J. Physiol. Heart Circ. Physiol., 292, H101–108.
Grivennikova, V. G., and Vinogradov, A. D. (2013) Partitioning of superoxide and hydrogen peroxide production by mitochondrial respiratory complex I, Biochim. Biophys. Acta, 1827, 446–454.
Brown, G. C., and Borutaite, V. (2012) There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells, Mitochondrion, 12, 1–4.
Starkov, A. A., Andreyev, A. Y., Zhang, S. F., Starkova, N. N., Korneeva, M., Syromyatnikov, M., and Popov, V. N. (2014) Scavenging of H2O2 by mouse brain mitochondria, J. Bioenerg. Biomembr., 46, 471–477.
Chouchani, E. T., Pell, V. R., Gaude, E., Aksentijevic, D., Sundier, S. Y., Robb, E. L., Logan, A., Nadtochiy, S. M., Ord, E. N., Smith, A. C., Eyassu, F., Shirley, R., Hu, C. H., Dare, A. J., James, A. M., Rogatti, S., Hartley, R. C., Eaton, S., Costa, A. S., Brookes, P. S., Davidson, S. M., Duchen, M. R., Saeb-Parsy, K., Shattock, M. J., Robinson, A. J., Work, L. M., Frezza, C., Krieg, T., and Murphy, M. P. (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS, Nature, 515, 431–435.
Kushnareva, Y., Murphy, A. N., and Andreyev, A. (2002) Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state, Biochem. J., 368 (Pt. 2), 545–553.
Ambrus, A., and Adam-Vizi, V. (2013) Molecular dynamics study of the structural basis of dysfunction and the modulation of reactive oxygen species generation by pathogenic mutants of human dihydrolipoamide dehydrogenase, Arch. Biochem. Biophys., 538, 145–155.
Ambrus, A., Tretter, L., and Adam-Vizi, V. (2009) Inhibition of the alpha-ketoglutarate dehydrogenase-mediated reactive oxygen species generation by lipoic acid, J. Neurochem., 109 (Suppl. 1), 222–229.
Kareyeva, A. V., Grivennikova, V. G., Cecchini, G., and Vinogradov, A. D. (2011) Molecular identification of the enzyme responsible for the mitochondrial NADH-supported ammonium-dependent hydrogen peroxide production, FEBS Lett., 585, 385–389.
Quinlan, C. L., Goncalves, R. L., Hey-Mogensen, M., Yadava, N., Bunik, V. I., and Brand, M. D. (2014) The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I, J. Biol. Chem., 289, 8312–8325.
Starkov, A. A., Fiskum, G., Chinopoulos, C., Lorenzo, B. J., Browne, S. E., Patel, M. S., and Beal, M. F. (2004) Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species, J. Neurosci., 24, 7779–7788.
Tahara, E. B., Barros, M. H., Oliveira, G. A., Netto, L. E., and Kowaltowski, A. J. (2007) Dihydrolipoyl dehydrogenase as a source of reactive oxygen species inhibited by caloric restriction and involved in Saccharomyces cerevisiae aging, FASEB J., 21, 274–283.
Tretter, L., and Adam-Vizi, V. (2004) Generation of reactive oxygen species in the reaction catalyzed by alphaketoglutarate dehydrogenase, J. Neurosci., 24, 7771–7778.
Zundorf, G., Kahlert, S., Bunik, V. I., and Reiser, G. (2009) α-Ketoglutarate dehydrogenase contributes to production of reactive oxygen species in glutamate-stimulated hippocampal neurons in situ, Neuroscience, 158, 610–616.
Pryde, K. R., and Hirst, J. (2011) Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer, J. Biol. Chem., 286, 18056–18065.
Treberg, J. R., Quinlan, C. L., and Brand, M. D. (2011) Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I), J. Biol. Chem., 286, 27103–27110.
Ohnishi, S. T., Shinzawa-Itoh, K., Ohta, K., Yoshikawa, S., and Ohnishi, T. (2010) New insights into the superoxide generation sites in bovine heart NADH-ubiquinone oxidoreductase (complex I): the significance of protein-associated ubiquinone and the dynamic shifting of generation sites between semiflavin and semiquinone radicals, Biochim. Biophys. Acta, 1797, 1901–1909.
Sled, V. D., Rudnitzky, N. I., Hatefi, Y., and Ohnishi, T. (1994) Thermodynamic analysis of flavin in mitochondrial NADH:ubiquinone oxidoreductase (complex I), Biochemistry, 33, 10069–10075.
Ohnishi, T. (1975) Thermodynamic and EPR characterization of iron-sulfur centers in the NADH-ubiquinone segment of the mitochondrial respiratory chain in pigeon heart, Biochim. Biophys. Acta, 387, 475–490.
Ohnishi, T. (1998) Iron-sulfur clusters/semiquinones in complex I, Biochim. Biophys. Acta, 1364, 186–206.
Ksenzenko, M., Konstantinov, A. A., Khomutov, G. B., Tikhonov, A. N., and Ruuge, E. K. (1983) Effect of electron transfer inhibitors on superoxide generation in the cytochrome bc1 site of the mitochondrial respiratory chain, FEBS Lett., 155, 19–24.
McLennan, H. R., and Degli Esposti, M. (2000) The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species, J. Bioenerg. Biomembr., 32, 153–162.
Ohnishi, T., and Trumpower, B. L. (1980) Differential effects of antimycin on ubisemiquinone bound in different environments in isolated succinate:cytochrome c reductase complex, J. Biol. Chem., 255, 3278–3284.
Quinlan, C. L., Orr, A. L., Perevoshchikova, I. V., Treberg, J. R., Ackrell, B. A., and Brand, M. D. (2012) Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions, J. Biol. Chem., 287, 27255–27264.
Siebels, I., and Drose, S. (2013) Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates, Biochim. Biophys. Acta, 1827, 1156–1164.
Miyadera, H., Shiomi, K., Ui, H., Yamaguchi, Y., Masuma, R., Tomoda, H., Miyoshi, H., Osanai, A., Kita, K., and Omura, S. (2003) Atpenins, potent and specific inhibitors of mitochondrial complex II (succinateubiquinone oxidoreductase), Proc. Natl. Acad. Sci. USA, 100, 473–477.
St-Pierre, J., Buckingham, J. A., Roebuck, S. J., and Brand, M. D. (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain, J. Biol. Chem., 277, 44784–44790.
Mills, E., and O’Neill, L. A. (2014) Succinate: a metabolic signal in inflammation, Trends Cell Biol., 24, 313–320.
Forman, H. J., and Kennedy, J. A. (1974) Role of superoxide radical in mitochondrial dehydrogenase reactions, Biochem. Biophys. Res. Commun., 60, 1044–1050.
Forman, H. J., and Kennedy, J. (1975) Superoxide production and electron transport in mitochondrial oxidation of dihydroorotic acid, J. Biol. Chem., 250, 4322–4326.
Dileepan, K. N., and Kennedy, J. (1985) Complete inhibition of dihydro-orotate oxidation and superoxide production by 1,1,1-trifluoro-3-thenoylacetone in rat liver mitochondria, Biochem. J., 225, 189–194.
Lakaschus, G., and Loffler, M. (1992) Differential susceptibility of dihydroorotate dehydrogenase/oxidase to brequinar sodium (NSC 368 390) in vitro, Biochem. Pharmacol., 43, 1025–1030.
Loffler, M., Becker, C., Wegerle, E., and Schuster, G. (1996) Catalytic enzyme histochemistry and biochemical analysis of dihydroorotate dehydrogenase/oxidase and succinate dehydrogenase in mammalian tissues, cells and mitochondria, Histochem. Cell Biol., 105, 119–128.
Hey-Mogensen, M., Goncalves, R. L., Orr, A. L., and Brand, M. D. (2014) Production of superoxide/H2O2 by dihydroorotate dehydrogenase in rat skeletal muscle mitochondria, Free Radic. Biol. Med., 72, 149–155.
Hail, N., Jr., Chen, P., Kepa, J. J., Bushman, L. R., and Shearn, C. (2010) Dihydroorotate dehydrogenase is required for N-(4-hydroxyphenyl)retinamide-induced reactive oxygen species production and apoptosis, Free Radic. Biol. Med., 49, 109–116.
Pinton, P., Rimessi, A., Marchi, S., Orsini, F., Migliaccio, E., Giorgio, M., Contursi, C., Minucci, S., Mantovani, F., Wieckowski, M. R., Del Sal, G., Pelicci, P. G., and Rizzuto, R. (2007) Protein kinase Cβ and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc, Science, 315, 659–663.
Giorgio, M., Migliaccio, E., Orsini, F., Paolucci, D., Moroni, M., Contursi, C., Pelliccia, G., Luzi, L., Minucci, S., Marcaccio, M., Pinton, P., Rizzuto, R., Bernardi, P., Paolucci, F., and Pelicci, P. G. (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis, Cell, 122, 221–233.
Gertz, M., Fischer, F., Leipelt, M., Wolters, D., and Steegborn, C. (2009) Identification of peroxiredoxin 1 as a novel interaction partner for the lifespan regulator protein p66Shc, Aging (Albany NY), 1, 254–265.
Banus, M. G. (1941) A design for a saturated calomel electrode, Science, 93, 601–602.
Zhen, Y., Hoganson, C. W., Babcock, G. T., and Ferguson-Miller, S. (1999) Definition of the interaction domain for cytochrome c on cytochrome c oxidase. I. Biochemical, spectral, and kinetic characterization of surface mutants in subunit ii of Rhodobacter sphaeroides cytochrome aa 3, J. Biol. Chem., 274, 38032–38041.
Gertz, M., and Steegborn, C. (2010) The lifespan-regulator p66Shc in mitochondria: redox enzyme or redox sensor? Antioxid. Redox Signal., 13, 1417–1428.
Galimov, E. R. (2010) The role of p66shc in oxidative stress and apoptosis, Acta Naturae, 2, 44–51.
Galimov, E. R., Chernyak, B. V., Sidorenko, A. S., Tereshkova, A. V., and Chumakov, P. M. (2014) Prooxidant properties of p66shc are mediated by mitochondria in human cells, PLoS One, 9, e86521.
Vogel, R., Wiesinger, H., Hamprecht, B., and Dringen, R. (1999) The regeneration of reduced glutathione in rat forebrain mitochondria identifies metabolic pathways providing the NADPH required, Neurosci. Lett., 275, 97–100.
Lopert, P., and Patel, M. (2014) Nicotinamide nucleotide transhydrogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system, J. Biol. Chem., 289, 15611–15620.
Freeman, H. C., Hugill, A., Dear, N. T., Ashcroft, F. M., and Cox, R. D. (2006) Deletion of nicotinamide nucleotide transhydrogenase: a new quantitative trait locus accounting for glucose intolerance in C57BL/6J mice, Diabetes, 55, 2153–2156.
Huang, T. T., Naeemuddin, M., Elchuri, S., Yamaguchi, M., Kozy, H. M., Carlson, E. J., and Epstein, C. J. (2006) Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase, Hum. Mol. Genet., 15, 1187–1194.
Chen, Y., Zhang, J., Lin, Y., Lei, Q., Guan, K. L., Zhao, S., and Xiong, Y. (2011) Tumor suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS, EMBO Rep., 12, 534–541.
Tao, R., Coleman, M. C., Pennington, J. D., Ozden, O., Park, S. H., Jiang, H., Kim, H. S., Flynn, C. R., Hill, S., Hayes McDonald, W., Olivier, A. K., Spitz, D. R., and Gius, D. (2010) Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress, Mol. Cell, 40, 893–904.
Tao, R., Vassilopoulos, A., Parisiadou, L., Yan, Y., and Gius, D. (2014) Regulation of MnSOD enzymatic activity by Sirt3 connects the mitochondrial acetylome signaling networks to aging and carcinogenesis, Antioxid. Redox Signal., 20, 1646–1654.
Radi, R., Turrens, J. F., Chang, L. Y., Bush, K. M., Crapo, J. D., and Freeman, B. A. (1991) Detection of catalase in rat heart mitochondria, J. Biol. Chem., 266, 22028–22034.
Salvi, M., Battaglia, V., Brunati, A. M., La Rocca, N., Tibaldi, E., Pietrangeli, P., Marcocci, L., Mondovi, B., Rossi, C. A., and Toninello, A. (2007) Catalase takes part in rat liver mitochondria oxidative stress defense, J. Biol. Chem., 282, 24407–24415.
Cadenas, E. (2004) Mitochondrial free radical production and cell signaling, Mol. Aspects Med., 25, 17–26.
Cox, A. G., Winterbourn, C. C., and Hampton, M. B. (2010) Mitochondrial peroxiredoxin involvement in antioxidant defense and redox signaling, Biochem. J., 425, 313–325.
Drechsel, D. A., and Patel, M. (2010) Respiration-dependent H2O2 removal in brain mitochondria via the thioredoxin/peroxiredoxin system, J. Biol. Chem., 285, 27850–27858.
Ho, Y. S., Magnenat, J. L., Bronson, R. T., Cao, J., Gargano, M., Sugawara, M., and Funk, C. D. (1997) Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia, J. Biol. Chem., 272, 16644–16651.
Cao, Z., Bhella, D., and Lindsay, J. G. (2007) Reconstitution of the mitochondrial PrxIII antioxidant defense pathway: general properties and factors affecting PrxIII activity and oligomeric state, J. Mol. Biol., 372, 1022–1033.
Trujillo, M., Clippe, A., Manta, B., Ferrer-Sueta, G., Smeets, A., Declercq, J. P., Knoops, B., and Radi, R. (2007) Pre-steady state kinetic characterization of human peroxiredoxin 5: taking advantage of Trp84 fluorescence increase upon oxidation, Arch. Biochem. Biophys., 467, 95–106.
Starkov, A. A. (2014) Measuring mitochondrial reactive oxygen species (ROS) production, in Systems Biology of Free Radicals and Antioxidants (Laher, I., ed.) Springer, Berlin-Heidelberg, pp. 265–278.
Starkov, A. A. (2010) Measurement of mitochondrial ROS production, Methods Mol. Biol., 648, 245–255.
Zhou, M., Diwu, Z., Panchuk-Voloshina, N., and Haugland, R. P. (1997) A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases, Anal. Biochem., 253, 162–168.
Cathcart, R., Schwiers, E., and Ames, B. N. (1983) Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay, Anal. Biochem., 134, 111–116.
LeBel, C. P., Ischiropoulos, H., and Bondy, S. C. (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress, Chem. Res. Toxicol., 5, 227–231
Brandt, R., and Keston, A. S. (1965) Synthesis of diacetyldichlorofluorescin: a stable reagent for fluorometric analysis, Anal. Biochem., 11, 6–9.
Keston, A. S., and Brandt, R. (1965) The fluorometric analysis of ultramicro quantities of hydrogen peroxide, Anal. Biochem., 11, 1–5.
Black, M. J., and Brandt, R. B. (1974) Spectrofluorometric analysis of hydrogen peroxide, Anal. Biochem., 58, 246–254.
Zheng, M., Aslund, F., and Storz, G. (1998) Activation of the OxyR transcription factor by reversible disulfide bond formation, Science, 279, 1718–1721.
Choi, H., Kim, S., Mukhopadhyay, P., Cho, S., Woo, J., Storz, G., and Ryu, S. E. (2001) Structural basis of the redox switch in the OxyR transcription factor, Cell, 105, 103–113.
Aslund, F., Zheng, M., Beckwith, J., and Storz, G. (1999) Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status, Proc. Natl. Acad. Sci. USA, 96, 6161–6165.
Lee, C., Lee, S. M., Mukhopadhyay, P., Kim, S. J., Lee, S. C., Ahn, W. S., Yu, M. H., Storz, G., and Ryu, S. E. (2004) Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path, Nat. Struct. Mol. Biol., 11, 1179–1185.
Belousov, V. V., Fradkov, A. F., Lukyanov, K. A., Staroverov, D. B., Shakhbazov, K. S., Terskikh, A. V., and Lukyanov, S. (2006) Genetically encoded fluorescent indicator for intracellular hydrogen peroxide, Nat. Methods, 3, 281–286.
Bilan, D. S., Pase, L., Joosen, L., Gorokhovatsky, A. Y., Ermakova, Y. G., Gadella, T. W., Grabher, C., Schultz, C., Lukyanov, S., and Belousov, V. V. (2013) HyPer-3: a genetically encoded H2O2 probe with improved performance for ratiometric and fluorescence lifetime imaging, ACS Chem. Biol., 8, 535–542.
Malinouski, M., Zhou, Y., Belousov, V. V., Hatfield, D. L., and Gladyshev, V. N. (2011) Hydrogen peroxide probes directed to different cellular compartments, PLoS One, 6, e14564.
Mishina, N. M., Tyurin-Kuzmin, P. A., Markvicheva, K. N., Vorotnikov, A. V., Tkachuk, V. A., Laketa, V., Schultz, C., Lukyanov, S., and Belousov, V. V. (2011) Does cellular hydrogen peroxide diffuse or act locally? Antioxid. Redox Signal., 14, 1–7.
Markvicheva, K. N., Bilan, D. S., Mishina, N. M., Gorokhovatsky, A. Y., Vinokurov, L. M., Lukyanov, S., and Belousov, V. V. (2011) A genetically encoded sensor for H2O2 with expanded dynamic range, Bioorg. Med. Chem., 19, 1079–1084.
Ermakova, Y. G., Bilan, D. S., Matlashov, M. E., Mishina, N. M., Markvicheva, K. N., Subach, O. M., Subach, F. V., Bogeski, I., Hoth, M., Enikolopov, G., and Belousov, V. V. (2014) Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide, Nat. Commun., 5, 5222.
Costa, A., Drago, I., Behera, S., Zottini, M., Pizzo, P., Schroeder, J. I., Pozzan, T., and Lo Schiavo, F. (2010) H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system, Plant J., 62, 760–772.
Elsner, M., Gehrmann, W., and Lenzen, S. (2011) Peroxisome-generated hydrogen peroxide as important mediator of lipotoxicity in insulin-producing cells, Diabetes, 60, 200–208.
Brejc, K., Sixma, T. K., Kitts, P. A., Kain, S. R., Tsien, R. Y., Ormo, M., and Remington, S. J. (1997) Structural basis for dual excitation and photoisomerization of the Aequorea victoria green fluorescent protein, Proc. Natl. Acad. Sci. USA, 94, 2306–2311.
Elsliger, M. A., Wachter, R. M., Hanson, G. T., Kallio, K., and Remington, S. J. (1999) Structural and spectral response of green fluorescent protein variants to changes in pH, Biochemistry, 38, 5296–5301.
Hanson, G. T., McAnaney, T. B., Park, E. S., Rendell, M. E., Yarbrough, D. K., Chu, S., Xi, L., Boxer, S. G., Montrose, M. H., and Remington, S. J. (2002) Green fluorescent protein variants as ratiometric dual emission pH sensors. 1. Structural characterization and preliminary application, Biochemistry, 41, 15477–15488.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Russian in Biokhimiya, 2015, Vol. 80, No. 5, pp. 612–630.
Rights and permissions
About this article
Cite this article
Andreyev, A.Y., Kushnareva, Y.E., Murphy, A.N. et al. Mitochondrial ROS metabolism: 10 Years later. Biochemistry Moscow 80, 517–531 (2015). https://doi.org/10.1134/S0006297915050028
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297915050028