Abstract
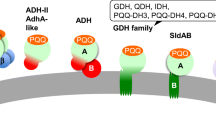
The quinate dehydrogenase (QDH) from Gluconobacter oxydans IFO3244 exhibits high affinity for quinate, suggesting its application in shikimate production. Nucleotide sequence analysis of the qdh gene revealed a full-length of 2475-bp encoding an 824-amino acid protein. The qdh gene has the unusual TTG translation initiation codon. Conserved regions and a signature sequence for the quinoprotein family were observed. Phylogenetic analysis demonstrated relatedness of QDH from G. oxydans to other quinate/shikimate dehydrogenases with the highest similarity (56%) with that of Acinetobacter calcoaceticus ADP1 and lower similarity (36%) with a membrane-bound glucose dehydrogenase of Escherichia coli. The function of the gene coding for QDH was confirmed by heterologous gene expression in pyrroloquinoline quinone-synthesizing Pseudomonas putida HK5.
Similar content being viewed by others
Abbreviations
- DAS:
-
dense alignment surface method
- mGDH:
-
membrane-bound glucose dehydrogenase
- PQQ:
-
pyrrolo-quinoline quinone
- QDH:
-
quinate dehydrogenase
References
Bongaerts, J., Kramer, M., Muller, U., Raeven, L., and Wubbolts, M. (2001) Metab. Eng., 3, 289–300.
Kim, C. U., Lew, W., Williams, M. A., Liu, H., Zhang, L., Swaminathan, S., Bischofberger, N., Chen, M. S., Mendel, D. B., Tai, C. Y., Laver, W. G., and Stevens, R. C. (1997) J. Am. Chem. Soc., 119, 681–690.
Rohloff, J. C., Kent, K. M., Postich, M. J., Becker, M. W., Chapman, H. H., Kelly, D. E., Lew, W., Louie, M. S., McGee, L. R., Prisbe, E. J., Schultze, L. M., Yu, R. H., and Zhang, L. (1998) J. Org. Chem., 63, 4545–4550.
Yi, J., Draths, K. M., Li, K., and Frost, J. W. (2003) Biotechnol. Prog., 19, 1450–1459.
Knop, D. R., Draths, K. M., Chandran, S. S., Barker, J. L., van Daeniken, R., Weber, W., and Frost, J. W. (2001) J. Am. Chem. Soc., 123, 10173–10182.
Adachi, O., Ano, Y., Toyama, H., and Matsushita, K. (2006) Biosci. Biotechnol. Biochem., 70, 2579–2582.
Adachi, O., Tanasupawat, S., Yoshihara, N., Toyama, H., and Matsushita, K. (2003) Biosci. Biotechnol. Biochem., 67, 2124–2131.
Ahmed, S. I., and Giles, N. H. (1969) J. Bacteriol., 99, 231–237.
Mitsuhashi, S., and Davis, B. D. (1954) Biochim. Biophys. Acta, 15, 268–280.
Ingledew, W. M., and Tai, C. C. (1972) Can. J. Microbiol., 18, 1817–1824.
Tresguerres, M. E. F., Torrontegui, G. D., and Canovas, J. L. (1970) Arch. Microbiol., 70, 110–118.
Whiting, G. C., and Coggins, R. A. (1967) Biochem. J., 102, 283–293.
Adachi, O., Yoshihara, N., Tanasupawat, S., Toyama, H., and Matsushita, K. (2003) Biosci. Biotechnol. Biochem., 67, 2115–2123.
Vangnai, A. S., Toyama, H., De-Eknamkul, W., Yoshihara, N., Adachi, O., and Matsushita, K. (2004) FEMS Microbiol. Lett., 241, 157–162.
Hanahan, D. (1983) J. Mol. Biol., 166, 557–580.
Toyama, H., Akiko, F., Matsushita, K., Shinakawa, E., Ameyama, M., and Adachi, O. (1995) J. Bacteriol., 177, 2442–2450.
Sambrook, J., and Russell, D. W. (2001) Molecular Cloning, A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor.
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994) Nucleic Acids Res., 22, 4673–4680.
Alako, B. T., Rainey, D., Nijveen, H., and Leunissen, J. A. (2006) Nucleic Acids Res., 34 (Web Server issue), W104–109.
Cserzo, M., Wallin, E., Simon, I., von Heijne, G., and Elofsson, A. (1997) Prot. Eng., 10, 673–676.
Kovach, M. E., Elzer, P. H., Hill, D. S., Robertson, G. T., Farris, M. A., Roop, R. M., 2nd, and Peterson, K. M. (1995) Gene, 166, 175–176.
Dully, J. R., and Grieve, P. A. (1975) Anal. Biochem., 64, 136–141.
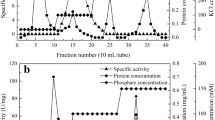
Kato, Y., and Asano, Y. (2003) Protein Exp. Purif., 28, 131–139.
Zhang, Q., Yan, X., Zhang, L., and Tang, W. (2006) Mol. Biol. (Moscow), 40, 418–424.
Gasteiger, E., Gattiker, A., Hoogland, C., Ivanyi, I., Appel, R. D., and Bairoch, A. (2003) Nucleic Acids Res., 31, 3784–3788.
Elsemore, D. A., and Ornston, L. N. (1994) J. Bacteriol., 176, 7659–7666.
Toyama, H., Mathews, F. S., Adachi, O., and Matsushita, K. (2004) Arch. Biochem. Biophys., 428, 10–21.
Felsenstein, J. (2002) PHYLIP (Phylogeny Inference Package) Version 3.6a3, Department of Genome Sciences, University of Washington, Seattle.
Quevillon, E., Silventoinen, V., Pillai, S., Harte, N., Mulder, N., Apweiler, R., and Lopez, R. (2005) Nucleic Acids Res., 33 (Web Server issue), W116–120.
Hulo, N., Bairoch, A., Bulliard, V., Cerutti, L., Cuche, B. A., Castro, E. D., Lachaize, C., Langendijk-Genevaux, P. S., and Sigrist, C. J. (2008) Nucleic Acids Res., 36, 1–5.
Cleton-Jansen, A. M., Dekker, S., van de Putte, P., and Goosen, N. (1991) Mol. Gen. Genet., 229, 206–212.
Duine, J. A., and Jongejan, J. A. (1989) Annu. Rev. Biochem., 58, 403–426.
Gallop, P. M., Paz, M. A., Fluckiger, R., and Kagan, H. M. (1989) Trends Biochem. Sci., 14, 343–346.
Inoue, T., Sunagawa, M., Mori, A., Imai, C., Fukuda, M., Takagi, M., and Yano, K. (1989) J. Bacteriol., 171, 3115–3122.
Yamada, M., Elias, M. D., Matsushita, K., Migita, C. T., and Adachi, O. (2003) Biochim. Biophys. Acta, 1647, 185–192.
Tonouchi, N., Tsuchida, T., Yoshinaga, F., Horinouchi, S., and Beppu, T. (1994) Biosci. Biotechnol. Biochem., 58, 1899–1901.
Tonouchi, N., Sugiyama, M., and Yokozeki, K. (2003) Biosci. Biotechnol. Biochem., 67, 211–213.
Marx, C. J., and Lidstrom, M. E. (2001) Microbiology, 147, 2065–2075.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2010, Vol. 75, No. 4, pp. 549–557.
Rights and permissions
About this article
Cite this article
Vangnai, A.S., Promden, W., De-Eknamkul, W. et al. Molecular characterization and heterologous expression of quinate dehydrogenase gene from Gluconobacter oxydans IFO3244. Biochemistry Moscow 75, 452–459 (2010). https://doi.org/10.1134/S0006297910040085
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297910040085