Abstract
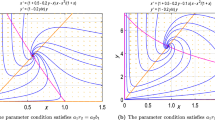
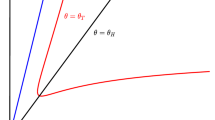
A time-continuous, spatially discrete motile predator–immobile prey model has been developed to describe the interactions between harpacticoid copepods and benthic microalgae in the intertidal zone. Harpacticoids perform periodic and nondirectional migrations; the local intensity of these migrations is trophic-dependent, related to the ratio of real to maximum food consumption rates. The zero-dimensional (non-spatial) analog of the model has three nontrivial equilibrium states, two of which are stable: one corresponds to overgrazing (permanently low abundance of prey controlled by hungry predator); the second is “welfare” (well-fed predator and resource-limited prey). Simulations show that in the spatially distributed model with close-to-real parameter values, two regimes can be realized: either total overgrazing or a persistent heterogeneous dynamic regime with short-lived patches of both populations, similar to that observed in nature. The predator’s ability to migrate narrows the “welfare” domain in parametric space, but increases the trophic efficiency of the system: in this domain, both average prey abundance and average consumption rate for the predator are higher than without migrations. The migrating gain is larger in an environment that is spatially heterogeneous for prey. Assumption of local awareness of the predator (preferred migrations toward a high abundance of prey) has no significant effect on the state of the system.











Similar content being viewed by others
REFERENCES
G. I. Abolmasova, “Feeding and the elements of energy balance of Gammarus olivii M.-Edw. from the Black Sea,” Biol. Morya (Kiev) 37, 41–45 (1976) [in Russian].
A. I. Azovsky and E. S. Chertoprud, “Spatio-temporal dynamics of the White Sea littoral harpacticoid community,” Oceanology (Engl. Transl.) 43, 103–111 (2003).
V. E. Zaika, Comparative Productivity of hydrobionts (Naukova Dumka, Kiev, 1983) [in Russian].
Yu. A. Mazei, I. V. Burkovskii, M. A. Saburova, et al., “Trophic structure of communities of psammophilic ciliates in the Chernaya River estuary,” Zool. Zh. 80 (11), 1283–1292 (2001) [in Russian, English summaty].
M. G. Sadovskii, M. Yu. Senashova, and P. A. Brychev, “A model of optimal migration of locally informed beings,” Dokl. Math. 80, 627–629 (2009).
M. Yu. Senashova and M. G. Sadovskii, “Modeling the dynamics of a two-species community under the optimization migration and local awareness of individuals,” Fundam. Issled., No. 3-1, 50–54 (2014).
L. M. Sushchenya, Respiration Intensity of Crustacean (Naukova Dumka, Kiev, 1972) [in Russian].
Yu. V. Tyutyunov, A. D. Zagrebneva, F. A. Surkov, and A. I. Azovsky, “Microscale patchiness of the distribution of copepods (Harpacticoida) as a result of trophotaxis,” Biophysics (Moscow) 54, 355–360 (2009).
Yu. V. Tyutyunov, A. D. Zagrebneva, F. A. Surkov, and A. I. Azovsky, “Modeling of the population density flow for periodically migrating organisms,” Oceanology (Engl. Transl.) 50, 67–76 (2010).
A. A. Udalov, I. V. Burkovskii, V. O. Mokievskii, et al., “Changes in the general characteristics of micro-, meio-, and macrobenthos along the salinity gradient in the White Sea estuary,” Oceanology (Engl. Transl.) 44, 514–525 (2004).
E. S. Chertoprud and A. I. Azovsky, “Seasonal dynamics of the populations of intertidal harpacticoids (Harpacticoida: Copepoda) in the White Sea,” Oceanology (Engl. Transl.) 46, 71–80 (2006).
E. S. Chertoprud, A. I. Azovsky, and F. V. Sapozhnikov, “Colonization of azoic sediments of different grain-size composition by littoral Harpacticoida: Copepoda,” Oceanology (Engl. Transl.) 45, 698–706 (2005).
T. S. Abu-Rezq, A. B. Yule, and S. K. Teng, “Ingestion, fecundity, growth rates and culture of the harpacticoid copepod, Tisbe furcata, in the laboratory,” Hydrobiologia 347 (1), 109–118 (1997).
W. Admiraal, “Tolerance of estuarine benthic diatoms to high concentrations of ammonia, nitrite ion, nitrate ion and orthophosphate,” Mar. Biol. 43 (4), 307–315 (1977).
W. Admiraal, H. Peletier, and H. Zomer, “Observations and experiments on the population dynamics of epipelic diatoms from an estuarine mudflat,” Estuarine, Coastal Shelf Sci. 14 (5), 471–487 (1982).
W. Admiraal and H. Peletier, “Influence of seasonal variations of temperature and light on the growth rate of cultures and natural populations of intertidal diatoms,” Mar. Ecol.: Prog. Ser. 2, 35–43 (1980).
C. M. V. Araujo-Castro and L. P. Souza-Santos, “Are the diatoms Navicula sp. and Thalassiosira fluviatilis suitable to be fed to the benthic harpacticoid copepod Tisbe biminiensis?” J. Exp. Mar. Biol. Ecol. 327 (1), 58–69 (2005).
A. I. Azovsky, E. S. Chertoprood, M. A. Saburova, and I. G. Polikarpov, “Spatio-temporal variability of micro-and meiobenthic communities in the White Sea intertidal sandflat,” Estuarine, Coastal Shelf Sci. 60 (4), 663–671 (2004).
A. I. Azovsky, M. A. Saburova, E. S. Chertoprood, and I. G. Polikarpov, “Selective feeding of littoral harpacticoids on diatom algae: hungry gourmands jump to survive?” in Proceedings of X European Ecological Congress (Eureco’05), Kusadasi, Turkey (META Press, Izmir, 2005), p. 96.
A. I. Azovsky, M. A. Saburova, E. S. Chertoprood, and I. G. Polikarpov, “Selective feeding of littoral harpacticoids on diatom algae: hungry gourmands?” Mar. Biol. 148, 327–337 (2005).
A. I. Azovsky, E. S. Chertoprood, M. A. Saburova, and I. G. Polikarpov, “Spatio-temporal variability of micro- and meiobenthic communities in the White Sea intertidal sandflat,” Estuarine, Coastal Shelf Sci. 60 (4), 663–671 (2004).
A. Basset, M. Fedele, and D. L. DeAngelis, “Optimal exploitation of spatially distributed trophic resources and population stability,” Ecol. Model. 151 (2–3), 245–260 (2002).
C. Bernstein, P. Auger, and J. Christophe, “Predator migration decisions, the ideal free distribution, and predator-prey dynamics,” Am. Nat. 153 (3), 267–281 (1999).
G. F. Blanchard, “Measurement of meiofauna grazing rates on microphytobenthos: is primary production a limiting factor?” J. Exp. Mar. Biol. Ecol. 147 (1), 37–46 (1991).
G. F. Blanchard, “Overlapping microscale dispersion patterns of meiofauna and microphytobenthos,” Mar. Ecol.: Prog. Ser. 68 (1–2), 101–111 (1990).
K. R. Carman, J. W. Fleeger, and S. M. Pomarico, “Response of a benthic food web to hydrocarbon contamination,” Limnol. Oceanogr. 42 (3), 561–571 (1997).
Decho A. W. and J. W. Fleeger, “Microscale dispersion of meiobenthic copepods in response to food-resource patchiness,” J. Exp. Mar. Biol. Ecol. 118 (3), 229–243 (1988).
J. W. Fleeger, M. A. Palmer, and E. B. Moser, “On the scale of aggregation of meiobenthic copepods on a tidal mudflat,” Mar. Ecol. 11 (3), 227–237 (1990).
J. W. Fleeger and A. W. Decho, “Spatial variability of interstitial meiofauna: a review,” Stygologia 3 (1), 35–54 (1987).
M. R. Garvie and M. Golinski, “Metapopulation dynamics for spatially extended predator–prey interactions,” Ecol. Complex 7 (1), 55–59 (2010).
M. P. Hassell, J. H. Lawton, and J. R. Beddington, “Sigmoid functional responses by invertebrate predators and parasitoids,” J. Anim. Ecol. 46 (1), 249–262 (1977).
C. Hauzy, M. Gauduchon, F. D. Hulot, and M. Loreau, “Density-dependent dispersal and relative dispersal affect the stability of predator-prey meta-communities,” J. Theor. Biol. 266 (3), 458–469 (2010).
G. R. Hicks, “The ecology of marine meiobenthic harpacticoid copepods,” Oceanogr. Mar. Biol. Ann. Rev. 21, 67–175 (1983).
R. Mac Nally, “Modeling confinement experiments in community ecology: differential mobility among competitors,” Ecol. Model. 129 (1), 65–85 (2000).
P. A. Montagna, “Rates of metazoan meiofaunal microbivory: a review,” Vie Milieu 45 (1), 1–10 (1995).
I. Noy-Meir, “Stability of grazing systems: an application of predator-prey graphs,” J. Ecol. 63 (2), 459–481 (1975).
J. Reiss and J. M. Schmid-Araya, “Feeding response of a benthic copepod to ciliate prey type, prey concentration and habitat complexity,” Freshwater Biol. 56 (8), 1519–1530 (2011).
G. Sach and H. Bernem, “Spatial patterns of Harpacticoida copepods on tidal flats,” Senchenberg. Mar. 26 (3–6), 97–10 (1996).
B. Sun and J. W. Fleeger, “Spatial and temporal patterns of dispersion in meiobenthic copepods,” Mar. Ecol.: Prog. Ser. 71, 1–11 (1991).
B. Sun, J. W. Fleeger, and R. S. Carney, “Sediment microtopography and the small-scale spatial distribution of meiofauna,” J. Exp. Mar. Biol. Ecol. 167 (1), 73–90 (1993).
R. B. Williams, “Division rates of salt marsh diatoms in relation to salinity and cell size,” Ecology 45 (4), 877–880 (1964).
D. R. Woods and J. H. Tietjen, “Horizontal and vertical distribution of meiofauna in the Venezuela Basin,” Mar. Geol. 68 (1–4), 233–241 (1985).
J. Yen, “Effects of prey concentration, prey size, predator life stage, predator starvation, and season on predation rates of the carnivorous copepod Euchaeta elongate,” Mar. Biol. 75 (1), 69–77 (1983).
V. E. Zaika, “Some remarks about primary production of benthic diatoms,” in Colloque Franco-Sovietique “Production Primaire et Secondaire” (Centre National pour l’Exploitation des Océans, Paris, 1980), Vol. 10.
Funding
The study was supported by the Russian Foundation for Basic Research (project nos. 17-04-00337, 18-04-00206).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Martynova
Rights and permissions
About this article
Cite this article
Smirnova, E.A., Azovsky, A.I. Modelling of Spatially Distributed Predator–Prey System with Periodically Migrating Predator (Case Study of the White Sea Intertidal Harpacticoids and Benthic Microalgae). Oceanology 60, 89–97 (2020). https://doi.org/10.1134/S000143702001021X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000143702001021X