Abstract
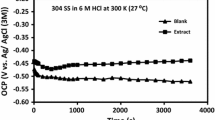
Here, by various electrochemical and analytical techniques, we have examined whether Eruca sativa aqueous extract can inhibit the corrosion of carbon steel in calcareous synthetic water. We detected differences in the morphology and phases of the films formed on the metallic surface under corrosion conditions. The electrochemical results suggest that, in the presence of E. sativa extract, the observed decrease in the corrosion current is associated with an increase in polarization resistance. EIS results reveal the presence of two time constants. The first one, at high frequency range (HF), is associated to the faradic process. The second one, at low frequency (LF), is related to the redox reaction occurring in the passive film. The highest inhibition efficiency, related to the large fraction of phenolic compounds present in the extract, can reach up to 95% with a dose of only 30 ppm.











Similar content being viewed by others
REFERENCES
Al-Otaibi, M.S., Al-Mayouf, A.M., Khan, M., Mousa, A.A., Al-Mazroa, S.A., and Alkhathlan, H.Z., Arabian J. Chem., 2014, vol. 7, p. 340.
Obot, I.B., Obi-Egbedi, N.O., and Umoren, S.A., Corros. Sci., 2009, vol. 51, p. 1868.
Yıldırım, A. and Cetin, M., Corros. Sci., 2008, vol. 50, p. 155.
Gentil, V., Corrosion, Rio de Janeiro: LTC, 2003.
El-Naggar, M.M., Corros. Sci., 2007, vol. 49, p. 2226.
Verna, C., Ebenso, E.E., and Quraishi, M.A., J. Mol. Liq., 2017, vol. 248, p. 927.
Negm, N.A., Kandile, N.G., Badr, E.A., and Mohammed, M.A., Corros. Sci., 2012, vol. 65, p. 94.
Lecante, A., Robert, F., Blandinières, P.A., and Roos, C., Curr. Appl. Phys., 2011, vol. 11, p. 714.
Umoren, S.A. and Ebenso, E.E., Pigm. Resin Technol., 2008, vol. 373, p. 173.
M’hiri, N., Veys-Renaux, D., Rocca, E., Ioannou, I., Boudhrioua, N.M., and Ghoul, M., Corros. Sci., 2016, vol. 102, p. 55.
Neske, A., Brandán, S.A., and Gervasi, C.A., J. Ind. Eng. Chem., 2018, vol. 58, p. 92.
Alibakhshi, E., Ramezanzadeh, M., Bahlakeh, G., Ramezanzadeh, B., and Motamedi, M., J. Mol. Liq., 2018, vol. 255, p. 185.
Loto, R.T., Results Phys., 2018, vol. 8, p. 172.
Tezeghdenti, M., Dhouibi, L., and Etteyeb, N., J. Bio-Tribo-Corros., 2015, p. 1.
El Ouariachi, E.M., Paolini, J., Bouklah, M., Elidrissi, A., Bouyanzar, A., Hammouti, B., Desjobert, J.M., and Costa, J., Acta Metall. Sin., 2010, vol. 23, p. 13.
Odiongenyi, A.O., Odoemelam, S.A., and Eddy, N.O., Port. Electrochim. Acta, 2009, vol. 27, p. 33.
Gerengi, H., Jazdzewska, A., and Kurtay, M., J. Adhes. Sci. Technol., 2015, vol. 29, p. 36.
Etteyeb, N. and Nóvoa, X.R., Corros. Sci., 2016, vol. 112, p. 471.
Yee, Y.J., Doctoral Thesis, Manchester: Institute of Science and Technology, 2004.
Pourriahi, M., Esfahani, M.N., and Motalebi, A., Surf. Eng. Appl. Electrochem., 2014, vol. 50, p. 525.
Shabani-Nooshabadi, M. and Ghandchi, M.S., J. Ind. Eng. Chem., 2015, vol. 31, p. 231.
Challouf, H., Souissi, N., Messaouda, M.B., Abidi, R., and Madani, A., J. Environ. Prot., 2016, vol. 7, p. 532.
Pradityana, A., Shahab, A., Noerochim, L., and Susanti, D., Int. J. Corros., 2016, vol.20, p. 1.
Omotoyinbo, J.A., Oloruntoba, D.T., and Olusegun, S.J., Int. J. Sci. Technol., 2013, vol. 2, p. 510.
Ben Amor, Y., Bousselmi, L., Takenouti, H., and Triki, E., Corros. Eng., Sci. Technol., 2005, vol. 40, p. 129.
Bousselmi, L., Fiaud, C., Tribollet, B., and Triki, E., Corros. Sci., 1977, vol. 39, p. 1711.
Bousselmi, L., Fiaud, C., Tribollet, B., and Triki, E., Electrochim. Acta, 1999, vol. 44, p. 4357.
Refait, Ph., Grolleau, A.-M., Jeannin, M., François, E., and Sabot, R., Corros. Sci., 2018, vol. 130, p. 76.
Wang, X. and Melchers, R.E., J. Loss Prev. Process Ind., 2017, vol. 45, p. 29.
Ben Amor, Y., Bousselmi, L., Tribollet, B., and Triki, E., Electrochim. Acta, 2010, vol. 55, p. 4820.
Hissel, J., Trib. Eau, 1998, vol. 51, p. 3.
Sadiq, A., Hayat, M.Q., and Mall, S.M., Nat. Prod. Chem. Res., 2014, vol. 2, p. 1.
Xiong, Q., Kadota, S., Tani, T., and Namba, T., Biol. Pharm. Bull., 1996, vol. 19, p. 1580.
Weckerle, B., Michel, K., Balázs, B., Schreier, P., and Tóth, G., Phytochemistry, 2001, vol. 57, p. 547.
Schulz, H. and Baranska, M., Vib. Spectrosc., 2007, vol. 43, p. 13.
Luz, B.R.D., New Phytol., 2006, vol. 172, p. 305.
Tezeghdenti, M., Etteyeb, N., Dhouibi, L., Kanoun, O., and Ammar Al-Hamri, A., Prot. Met. Phys. Chem. Surf., 2017, vol. 53, p. 753.
Zhou, X., Yang, H., and Wang, F., Corros. Sci., 2012, vol. 54, p.193.
Singh, A.K. and Quraishi, M.A., Corros. Sci., 2010, vol. 52, p. 1373.
El-Taib Heakal, F., Deyab, M.A., Osman, M.M., and Elkholy, A.E., Desalination, 2018, vol. 425, p. 111.
Valcarce, M.B. and Vázquez, M., Corros. Sci., 2010, vol. 52, p. 1413.
Zhang, B., He, Ch., Wang, Ch., Sun, P., Li, F., and Lin, Y., Corros. Sci., 2015, vol. 94, p. 6.
Guitián, B., Nóvoa, X.R., and Puga, B., Electrochim. Acta, 2011, vol. 56, p. 7772.
Andrade, C., Merino, P., Nóvoa, X.R., Pérez, M.C., and Soler, L., Mater. Sci. Forum, 1995, vol. 192, p. 891.
Malinovschi, V., Ducu, C., Aldea, N., and Fulger, M., J. Nucl. Mater., 2006, vol. 352, p. 107.
Cui, H., Liu, Y., and Ren, W., Adv. Powder Technol., 2013, vol. 24, p. 93.
Kontoyannis, C.G. and Vagenas, N.V., Analyst, 2000, vol. 125, p. 251.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wahiba Ebdelly, Ben Hassen, S., Nóvoa, X.R. et al. Inhibition of Carbon Steel Corrosion in Neutral Calcareous Synthetic Water by Eruca sativa Extract. Prot Met Phys Chem Surf 55, 591–602 (2019). https://doi.org/10.1134/S2070205119030110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205119030110