Abstract
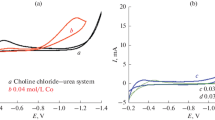
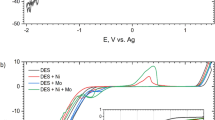
Ternary Zn–Mn–Ni alloy coatings were electrodeposited for the first time from a choline chloride based ionic liquid with the aim of collecting properties of binary Zn–Mn and Zn–Ni alloys into one alloy system. The effect of electrodeposition potential on the composition and corrosion performance of the obtained ternary Zn–Mn–Ni deposits was investigated and contrasted with the characteristics of Zn–Mn and Zn–Ni deposits. Cyclic voltammetry revealed that the deposition of ternary Zn–Mn–Ni alloys behaved differently from the deposition of binary Zn–Mn and Zn–Sn alloys and that Mn deposition takes place at positive potentials in the Zn–Mn–Ni electrolyte than in the Zn–Mn electrolyte due to the presence of Ni2+ ions in the electrolyte. X-ray diffraction studies showed that the Zn–Mn–Ni ternary alloys consist of a lattice of Zn (with Mn and Ni imbedded inside) at low electrodeposition potentials and MnZn(with Ni imbedded inside) phase at high electrodeposition potentials. Chemical composition analysis show that the Mn content in the ternary Zn–Mn–Ni alloy increased with increase in electrodeposition potential, whereas Zn and Ni contents are suppressed. The corrosion tests results indicate that through addition of Ni into the Zn–Mn binary alloy, the Zn–Mn–Ni alloy tailored are more corrosion resistant than the Zn–Mn binary alloy whilst the passivation behavior is still preserved.
Similar content being viewed by others
References
Bučko, M., Rogan, J., Stevanović, S.I., et al., Corros. Sci., 2011, vol. 53, p. 2861.
Veeraraghavan, B., Haran, B., Kumaraguru, S., and Popov, B., J. Electrochem. Soc., 2003, vol. 150, p. B131.
Kumaraguru, S., Veeraraghavan, B., and Popov, B., J. Electrochem. Soc., 2006, vol. 153, p. B253.
Boshkov, N., Surf. Coat. Technol., 2003, vol. 172, p. 217.
Bozzini, B., Griskonis, E., and Fanigliulo, A., Surf. Coat. Technol., 2002, vol. 154, p. 294.
Boshkov, N., Petrov, K., Kovacheva, D., et al., Electrochim. Acta, 2005, vol. 51, p. 77.
Srinivasan, K.N., Selvam, M., and Iyer, S.V.K., J. Appl. Electrochem., 1993, vol. 23, p. 358.
Kayshap, R., Srivastava, S.N., and Srivastava, S.C., J. Appl. Electrochem., 1985, vol. 15, p. 23.
Endres, F., Chem. Phys., 2002, vol. 3, p. 144.
Endres, F. and El Abedin, S.Z., Phys. Chem. Chem. Phys., 2006, vol. 8, p. 2116.
Kim, Y.S., Choi, W.Y., Jang, K.P., et al., Fluid Phase Equilib., 2005, vol. 228, p. 439.
Chen, P.Y. and Hussey, C.L., Electrochim. Acta, 2007, vol. 52, p. 1857.
Sylla, D., Savall, C., Gadouleau, M., et al., Surf. Coat. Technol., 2005, vol. 200, p. 2137.
Basavanna, S. and Naik, Y.A., J. Appl. Electrochem., 2009, vol. 39, p. 1975.
Hegde, A.C., Venkatakrishna, K., and Eliaz, N., Surf. Coat. Technol., 2010, vol. 205, p. 2031.
Bajat, J.B., Petrović, A.B., and Maksimović, M.D., J. Serb. Chem. Soc., 2005, vol. 70, p. 1427.
Gavrila, M., Millet, J., Mazille, H., et al., Surf. Coat. Technol., 2000, vol. 123, p. 164.
Alfantazi, A.M., Page, J., and Erb, U., J. Appl. Electrochem., 1996, vol. 26, p. 1225.
Fashu, S., Gu, C.D., Zhang, J.L., et al., J. Mater. Eng. Perform., 2014, vol. 24, p. 434.
Fashu, S., Gu, C.D., Wang, X.L., and Tu, J.P., Surf. Coat. Technol., 2014, vol. 242, p. 34.
Patterson, A.L., Phys. Rev., 1939, vol. 56, p. 978.
Hosseini, M.G., Ashassi-Sorkhabi, H., and Ghiasvand, H.A.Y., Surf. Coat. Technol., 2008, vol. 202, p. 2897.
Nasirpouri, F., Sanaeian, M.R., Samardak, A.S., et al., Appl. Surf. Sci., 2014, vol. 292, p. 795.
Liang, J.L., Du, Y., Liao, C.Z., et al., J. Alloys Compd., 2010, vol. 489, p. 362.
Ganesan, S., Prabhu, G., and Popov, B.N., Surf. Coat. Technol., 2014, vol. 238, p. 143.
Müller, C., Sarret, M., and Andreu, T., J. Electrochem. Soc., 2002, vol. 149, p. C600.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Fashu, S., Khan, R. Studies on electrochemical deposition of novel Zn–Mn–Ni ternary alloys from an ionic liquid based on choline chloride. Prot Met Phys Chem Surf 53, 118–126 (2017). https://doi.org/10.1134/S2070205117010051
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205117010051