Abstract
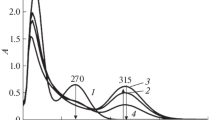
The kinetics of decomposition of thiourea and N-methylthiourea S,S-dioxides in alkaline aqueous medium and their reactions with oxygen, Indigo Carmine, and Acid Yellow 11 were studied. The decomposition of thiourea S,S-dioxides in weakly alkaline media was found to follow two pathways leading to the formation of ammonia and sulfoxylate ion, respectively. The second pathway predominates in strongly alkaline media. Thiourea S,S-dioxides can be used for selective reduction of azo compounds to the corresponding hydrazo derivatives.
Similar content being viewed by others
References
Budanov, V.V., Khimiya i tekhnologiya vosstanovitelei na osnove sul’foksilovoi kisloty. Rongalit i ego analogi (Chemistry and Technology of Reducing Agents Based on Sulfoxylic Acid. Rongalite and Its Analogs), Moscow: Khimiya, 1984.
Polenov, Yu.V., Cand. Sci. (Chem.) Dissertation, Ivanovo, 1985.
Davtyan, K.A. and Makarov, S.V., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2001, vol. 44, no. 3, p. 103.
Budanov, V.V. and Makarov, S.V., Khimiya serosoderzhashchikh vosstanovitelei (Chemistry of Sulfurcontaining Reducing Agents), Moscow: Khimiya, 1994.
Makarov, S.V., Usp. Khim., 2001, vol. 70, no. 10, p. 995.
Miller, A.E., Bischoff, J.J., and Pae, K., Chem. Res. Toxicol., 1988, vol. 1, no. 3, p. 169.
Makarov, S.V., Kudrik, E.V., van Eldik, R., and Naidenko, E.V., J. Chem. Soc., Dalton Trans., 2002, no. 22, p. 4074.
Huang, S.-L. and Chen, T.Y., J. Chin. Chem. Soc., 1975, vol. 22, no. 1, p. 91.
Louis-Andre, O. and Gelbard, G., Bull. Soc. Chim. Fr., 1986, no. 4, p. 565.
Nesmeyanov, A.N. and Nesmeyanov, N.A., Nachala organicheskoi khimii (Principles of Organic Chemistry), Moscow: Khimiya, 1974, vol. 2.
Walter, W. and Randau, G., Justus Liebigs Ann. Chem., 1969, vol. 722, p. 80.
Stepanov, B.I., Vvedenie v khimiyu i tekhnologiyu organicheskikh krasitelei (Chemistry and Technology of Organic Dyes. An Introduction), Moscow: Khimiya, 1977, p. 279.
Tietze, L.-F. and Eicher, T., Reactions and Syntheses in the Organic Chemistry Laboratory, Mill Valley, CA: Univ. Science Books, 1989. Translated under the title Preparativnaya organicheskaya khimiya, Moscow: Mir, 1999, p. 389.
Svarovsky, S.A., Simoyi, R.H., and Makarov, S.V., J. Chem. Soc., Dalton Trans., 2000, no. 4, p. 511.
Debš, D., Electrochemical Data, Budapest: Akad. Kiado, 1978.
Author information
Authors and Affiliations
Additional information
Original Russian Text © S.V. Makarov, E.V. Kudrik, K.A. Davydov, 2006, published in Zhurnal Obshchei Khimii, 2006, Vol. 76, No. 10, pp. 1669–1673.
Rights and permissions
About this article
Cite this article
Makarov, S.V., Kudrik, E.V. & Davydov, K.A. Reaction of thiourea S,S-dioxides with dyes containing carbonyl or azo groups. Russ J Gen Chem 76, 1599–1603 (2006). https://doi.org/10.1134/S1070363206100173
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1070363206100173