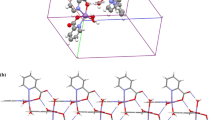
Abstract
A novel trinuclear manganese(II) complex, Mn3(C12H8N2)2(C10H11O5)6 (I), has been hydrothermally synthesized and characterized by single-crystal X-ray diffraction. The crystal belongs to the monoclinic system, space group P21/n, with cell parameters a = 12.1935(7), b = 19.0249(11), c = 18.0515(10)Å, β = 105.0430(10)°, V = 4044.1(4)Å3, Z = 2. Two of the three manganese atoms in the crystal structure are crystallographically equivalent and adopt N2O4 coordination mode, whereas the remaining manganese atom adopts O6 coordination mode, which forms a nearly regular octahedron. The experimental result of thermal analysis shows that complex I remains stable below 300°C.
Similar content being viewed by others
References
Shaikh, N., Goswami, S., Panja, A., et al., Inorg. Chem., 2004, vol. 43, p. 5908.
Fan, Y., Li, G.H., Shi, Z., et al., J. Solid State Chem., 2004, vol. 177, p. 4346.
Liu, X.Y., Riera, V., Ruiz, M.A., et al., Organometallics, 1996, vol. 15, p. 974.
Wang, X.Y., Gan, L., Zhang, S.W., et al., Inorg. Chem., 2004, vol. 43, p. 4615.
Ma, S.Q. and Zhou, H.C., Chem. Commun., 2010, vol. 1, p. 44.
Jiang, D.M., Mallat, T., Meier, D.M., et al., J. Catal., 2009, vol. 270, p. 26.
Stock, N., Rauscher, M., and Bein, T., J. Solid State Chem., 2004, vol. 177, p. 642.
Bai, Y., Gao, H., Dang, D.B., et al., J. Mol. Struct., 2009, vol. 934, p. 53.
Nicolau, M.P.M., Bárcia, P., Gallegos, J.M., et al., J. Phys. Chem., C, 2009, vol. 113, p. 13173.
Amel’chenkova, E.V., Denisova, T.O., and Nefedov, S.E., Mendeleev Commun., 2004, vol. 14, p. 103.
Terazzi, E., Bourgogne, C., Welter, R., et al., Angew. Chem. Int. Ed., 2008, vol. 47, p. 490.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, vol. 46, p. 467.
Sheldrick, G.M., SHELXS-97, Göttingen (Germany): Univ. of Göttingen, 1997.
Sheldrick, G.M., SHELX-97, Göttingen (Germany): Univ. of Göttingen, 1997.
Kumari, N., Prajapati, R., and Hayashidab, O., Polyhedron, 2010, vol. 29, p. ?. 2758.
Wang, R.H., Gao, E.Q., and Hong, M.C., Inorg. Chem., 2003, vol. 42, p. 5487.
Ji, J.W. and Han, Z.B., Russ. J. Coord. Chem., 2010, vol. 36, p. 41.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Zhang, L., Wang, C., Wu, X. et al. Hydrothermal synthesis, crystal structure, and thermal analysis of a Novel trinuclear manganese complex: Mn3(C12H8N2)2(C10H11O5)6 . Russ J Coord Chem 37, 382–387 (2011). https://doi.org/10.1134/S1070328411040129
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328411040129