Abstract
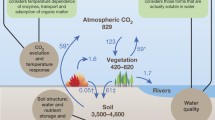
The main sources of the low-molecular-weight aliphatic and aromatic organic acids (LMWOAs) in soils are plant residues, root exudates, and microbial metabolites. In the solutions of upper soil layers of forest ecosystems, citric, oxalic, and malic acids predominate among aliphatic LMWOAs, while substituted and unsubstituted benzoic and cinnamic acids predominate among aromatic LMWOAs. The LMWOA concentrations in plant tissues, root exudates, and microbial metabolites vary from several to some tens of millimoles per liter. In soil solutions, these values decrease by one–two orders of magnitude because of LMWOA sorption and biodegradation, their migration downward the profile, and dilution effect. The sorption of LMWOA anions increases with a decrease in pH and an increase in the concentrations of iron oxides/hydroxides and amorphous aluminosilicate clays. Polybasic acids are more tightly bound to solid phase as compared with monobasic acids. The sorption mechanisms vary depending on pH and LMWOA molecular structure and include formation of innersphere and outersphere surface complexes, ligand exchange, and cation bridge bond. Aromatic LMWOAs can be involved in a hydrophobic interaction. LMWOAs are very rapidly decomposed in the upper horizons, their lifespan amounting to several hours. Some important functions of LMWOAs in soils and landscapes are discussed, including their involvement in the global carbon cycle, mineral weathering, Al and Fe mobilization and translocation, mobilization of Fe and P compounds in the soils deficient in these elements, formation of acid–base buffering of natural waters, and detoxication of soils with increased contents of aluminum, heavy metals, and organic pollutants in soil solutions.
Similar content being viewed by others
Notes
Soils and horizons are named in author’s edition.
REFERENCES
N. A. Artemkina, T. T. Gorbacheva, and N. V. Lukina, “Low-molecular-weight organic acids in soil water of forests of the Kola Peninsula,” Lesovedenie, No. 6, 37–44 (2008).
E. I. Karavanova, “Dissolved organic matter: fractional composition and sorbability by the soil solid phase (review of literature),” Eurasian Soil Sci. 46, 833–844 (2013).
I. S. Kaurichev and E. M. Nozdrunova, “Migration and qualitative composition of water-soluble organic matter in forest-meadow soils,” Izv. Timiryazevsk. S-kh. Akad., No. 5, 91–106 (1962).
I. S. Kaurichev, T. N. Ivanova, and E. M. Nozdrunova, “The content of low-molecular organic acids in water-soluble organic soil matter,” Pochvovedenie, No. 3, 27–35 (1963).
I. S. Kaurichev and L. N. Frolova, “Individual water-soluble organic matter in forest litter,” Dokl. Timiryazevsk. S-kh. Akad., No. 115, 19–24 (1965).
I. S. Kaurichev, et al., “Qualitative composition of water-soluble organic matter extracted from humificated and non-humificated forest litters,” Izv. Timiryazevsk. S-kh. Akad., No. 2, 100–109 (1972).
M. S. Malinina and S. V. Ivanilova, “Phenol compounds in solutions of soils of different types in the central forest state biosphere reserve,” Eurasian Soil Sci. 41, 377–385 (2008).
G. V. Motuzova, A. V. Zorina, and A. A. Stepanov, “Water-soluble organic substances in litters of Al–Fe–humus podzols of the Kola Peninsula,” Eurasian Soil Sci. 38, 57–64 (2005).
D. S. Orlov, Soil Humic Acids and General Theory of Humification (Moscow State Univ., Moscow, 1990) [in Russian].
T. A. Sokolova, I. I. Tolpeshta, L. V. Lysak, Yu. A. Zavgorodnyaya, T. S. Chalova, M. M. Karpukhin, and Yu. G. Izosimova, “Biological characteristics and concentrations of mobile Fe, Al, Si compounds in spruce rhizosphere in podzolic soil,” Eurasian Soil Sci. 51, 1317–1325 (2018).
E. A. Timofeeva, Candidate’s Dissertation in Biology (Moscow, 2010).
I. M. Yashin and I. S. Kaurichev, “The role of low-molecular organic acids in the abiogenic transformation of humic substances in soils of the taiga-forest zone,” Izv. Timiryazevsk. S-kh. Akad., No. 5, 36–49 (1992).
I. M. Yashin and I. S. Kaurichev, “Pedogenic functions of water-soluble organic substances in taiga landscapes,” Pochvovedenie, No. 10, 49–61 (1992).
I. M. Yashin, L. L. Shishov, and V. A. Raskatov, Analysis of Migration of Substances (Timiryazev Agricultural Academy, Moscow, 2001) [in Russian].
J. Abadía, A.-F. López-Millán, R. Adamo, and A. Anunciacin, “Organic acids and Fe deficiency: a review,” Plant Soil 241, 75–86 (2002).
G. Angst, I. Kögel-Knabner, K. Kirfel, D. Hertel, and C. W. Mueller, “Spatial distribution and chemical composition of soil organic matter fractions in rhizosphere and non-rhizosphere soil under European beech (Fagus sylvatica L.),” Geoderma 264, 179–187 (2016).
V. L. Bailey, A. P. Smith, M. Tfaily, S. J. Fansler, and B. Bond-Lamberty, “Differences in soluble organic carbon chemistry in pore waters sampled from different pore size domains,” Soil Biol. Biochem. 107, 133–143 (2017).
C. Balland, A. Poszwa, C. Leyval, and C. Mustin, “Dissolution rates of phyllosilicates as a function of bacterial metabolic diversity,” Geochim. Cosmochim. Acta 74, 5478–5493 (2010).
Z. Balogh-Brunstad, C. K. Keller, T. J. Dickinson, F. Stevens, C. Y. Li, and B. T. Bormann, “Biotite weathering and nutrient uptake by ectomycorrhizal fungus, Suillus tomentosus, in liquid-culture experiments,” Geochim. Cosmochim. Acta 72, 2601–2618 (2008).
J. Barceló and C. Poschenrieder, “Fast root growth responses, root exudates, internal detoxification as clues to the mechanisms of aluminum toxicity and resistance: a review,” Environ. Exp. Bot. 48, 75–92 (2002).
R. Baziramakenga, R. R. Simard, and G. D. Leroux, “Determination of organic acids in soil extracts by ion chromatography,” Soil Biol. Biochem. 27 (3), 349–356 (1995).
P. C. Bennet, M. E. Melcer, D. I. Siegel, and J. P. Hasset, “The dissolution of quartz in dilute aqueous solutions of organic acids at 25°C,” Geochim. Cosmochim. Acta 52 (6), 1521–1530 (1988).
P. C. Bennet, “The dissolution of quartz in organic rich aqueous systems,” Geochim. Cosmochim. Acta 55 (7), 1781–1797 (1991).
A. Bergelin, P. A. W. van Hees, O. Wahlberg, and U. S. Lundström, “The acid-base properties of high and low molecular weight organic acids in soil solutions of podzolic soils,” Geoderma 94, 223–235 (2000).
J. S. Bhatti, N. B. Comerford, and C. T. Johnston, “Influence of oxalate and soil organic matter on sorption and desorption of phosphate onto a spodic horizon,” Soil Sci. Soc. Am. J. 62, 1089–1095 (1989).
P. Cambier and G. Sposito, “Adsorption of citric acid by synthetic pseudobemite,” Clays Clay Miner. 39 (4), 369–374 (1991).
B. Chefetz, S. Eldad, and T. Polubesova, “Interactions of aromatic acids with montmorillonite: Ca2+- and Fe3+-saturated clays versus Fe3+–Ca2+-clay system,” Geoderma 160, 608–613 (2011).
Y. Chen, M. A. Glaus, L. R. van Loon, and U. Mäder, “Transport of low molecular weight organic compounds in compacted illite and kaolinite,” Chemosphere 198, 226–237 (2018).
P. K. F. Chin and G. L. Mills, “Kinetics and mechanisms of kaolinite dissolution: effects of organic ligands,” Chem. Geol. 90, 307–317 (1991).
M. Corvasce, A. Zsolnay, V. D. Orazio, R. Lopez, and T. M. Miano, “Characterization of water extractable organic matter in a deep soil profile,” Chemosphere 62, 1583–1590 (2006).
P.-E. Courty, M. Buee, A. G. Diedhiou, P. Frey-Klett, F. LeTacon, F. Rineau, M.-P. Turpault, S. Uroz, and J. Garbaye, “The role of ectomycorrhizal communities in forest ecosystem processes: new perspectives and emerging concepts,” Soil Biol. Biochem. 42, 679–698 (2010).
K. Cromack, P. Sollins, W. C. Graustein, K. Speidel, A. W. Todd, G. Spycher, C. Y. Li, and R. L. Todd, “Calcium oxalate accumulation and soil weathering in mats of the hypogeous fungus Hysterangium crassum,” Soil Biol. Biochem. 11, 463–468 (1979).
F. D. Dakora and D. A. Phillips, “Root exudates as mediators of mineral acquisition in low-nutrient environments,” Plant Soil 245, 35–47 (2002).
F. A. Dijkstra, C. Geibe, S. Holmström, U. S. Lundström, and N. van Breemen, “The effect of organic acids on base cation leaching from the forest floor under six North American tree species,” Eur. J. Soil Sci. 52, 205–214 (2001).
Dubus I.G., Barriuso E., and Calvet, R. Sorption of weak organic acids in soils: clofencet, 2,4-D, salicylic acid,” Chemosphere 45, 767–774 (2001).
M. Duputel, N. Devau, M. Brossard, B. Jaillard, D. L. Jones, P. Hinsinger, and F. Gérard, “Citrate adsorption can decrease soluble phosphate concentration in soils: results of theoretical modeling,” Appl. Geochem. 35, 120–131 (2013).
S. B. Durman, A. B. Menendez, and A. M. Godeas, “Variation in oxalic acid production and mycelial compatibility within field populations of Sclerotinia sclerotiorum,” Soil Biol. Biochem. 37, 2180–2184 (2005).
M. E. Essington, Soil and Water Chemistry (CRC Press, Boca Raton, 2004).
H. Fischer, J. Ingwersen, and Y. Kuzyakov, “Microbial uptake of low-molecular-weight organic substances out-competes sorption in soil,” Eur. J. Soil Sci. 61, 504–513 (2010).
T. R. Fox and N. B. Comerford, “Low-molecular-weight organic acids in selected forest soils of the southeastern USA,” Soil Sci. Soc. Am. J. 54 (4), 1139–1144 (1990).
K. Fujii, M. Aoki, and K. Kitayama, “Biodegradation of low molecular weight organic acids in rhizosphere soils from a tropical montane rain forest,” Soil Biol. Biochem. 47, 142–148 (2012).
K. Fujii, K. Morioka, R. Hangs, S. Funakawa, T. Kosaki, and D. W. Anderson, “Rapid turnover of organic acids in a Dystric Brunisol under a spruce–lichen forest in northern Saskatchewan, Canada,” Can. J. Soil Sci. 93, 295–304 (2013).
G. Furrer and W. Stumm, “The role of surface coordination in the dissolution of δ-Al2O3 in dilute acids,” Chimia 37, 338–341 (1983).
G. Furrer and W. Stumm, “The coordination chemistry of weathering. I. Dissolution kinetics of δ-Al2O3 and BeO,” Geochim. Cosmochim. Acta 50, 1847–1860 (1986).
C. Gallet and F. Pellissier, “Phenolic compounds in natural solutions of a coniferous forest,” J. Chem. Ecol. 23 (10), 2401–2412 (1997).
S. Gangloff, P. Stille, M.-C. Pierret, T. Weber, and F. Chabaux, “Characterization and evolution of dissolved organic matter in acidic forest soil and its impact on the mobility of major and trace elements (case of the Strengbach watershed),” Geochim. Cosmochim. Acta 130, 21–41 (2014).
R. P. Griffiths, J. E. Baham, and B. A. Caldwell, “Soil solution chemistry of ectomycorrhizal mats in forest soil,” Soil Biol. Biochem. 26, 331–337 (1994).
G. Guggenberger and W. Zech, “Composition and dynamics of dissolved carbohydrates and lignin-degradation products in two coniferous forests in N. E. Bavaria, Germany,” Soil Biol. Biochem. 26 (1), 19–27 (1994).
K. Hanna, “Sorption of two aromatic acids onto iron oxides: experimental study and modeling,” J. Colloid Interface Sci. 309, 419–428 (2007).
K. Hanna and C. Carteret, “Sorption of 1-hydroxy-2-naphthoic acid to goethite, lepidocrocite and ferrihydrite: batch experiments and infrared study,” Chemosphere 70, 178–186 (2007).
J. Z. He, A. De Cristofaro, and A. Violante, “Comparison of adsorption of phosphate, tartrate, and oxalate on hydroxy aluminum montmorillonite complexes,” Clays Clay Miner. 47 (2), 226–233 (1999).
E. Hoffland, R. van den Boogaard, J. Nelemans, and G. Findenegg, “Biosynthesis and root exudation of citric and malic acids in phosphate-starved rape plants,” New Phytol. 22, 675–680 (1992).
Q. Huang, Z. Zhao, and W. Chen, “Effects of several low-molecular weight organic acids and phosphate on the adsorption of acid phosphatase by soil colloids and minerals,” Chemosphere 52, 571–579 (2003).
W. H. Huang and W. D. Keller, “Dissolution of rock-forming minerals in organic acids: simulated first stage weathering of fresh mineral surface,” Am. Miner. 55 (11–12), 2076–2094 (1970).
W. H. Huang and W. D. Keller, “Dissolution of clay minerals in dilute organic acids at room temperature,” Am. Miner. 56 (5–6), 1082–1095 (1971).
N. V. Hue, G. R. Craddock, and F. Adams, “Effect of organic acids on aluminum toxicity in subsoils,” Soil Sci. Soc. Am. J. 50 (1), 28–34 (1986).
B. W. Hütsch, J. Augustin, and W. Merbach, “Plant rhizodeposition—an important source for carbon turnover in soils,” J. Plant Nutr. Soil Sci. 165, 397–407 (2002).
S. Jagadamma, M. A. Mayes, Y. L. Zinn, G. Gísladóttir, and A. E. Russell, “Sorption of organic carbon compounds to the fine fraction of surface and subsurface soils,” Geoderma 213, 79–86 (2014).
Inderjit and P. C., Bhowmik, “Sorption of benzoic acid onto soil colloids and its implications for allelopathy studies,” Biol. Fertil. Soils 40, 345–348 (2004).
D. L. Jones, “Organic acids in the rhizosphere—a critical review,” Plant Soil 205, 25–44 (1998).
D. L. Jones and D. S. Brassington, “Sorption of organic acids in acid soils and its implications in the rhizosphere,” Eur. J. Soil Sci. 49, 447–455 (1998).
D. L. Jones, P. G. Dennis, A. G. Owen, and P. A. W. van Hees, “Organic acid behavior in soils—misconceptions and knowledge gaps,” Plant Soil 248, 31–41 (2003).
K. Kaiser, M. Kaupenjohann, and W. Zech, “Sorption of dissolved organic carbon in soils: effects of soil sample storage, soil-to-solution ratio, temperature,” Geoderma 99, 317–328 (2001).
A. J. Krzyszowska, M. J. Blaybock, G. F. Vance, and M. B. David, “Ion-chromatographic analysis of low molecular weight organic acids in Spodosol forest floor solution,” Soil Sci. Soc. Am. J. 60 (5), 1565–1571 (1996).
J. D. Kubicki, D. Tunega, and S. Kraemer, “A density functional theory investigation of oxalate and Fe(II) adsorption onto the (010) goethite surface with implications for ligand- and reduction-promoted dissolution,” Chem. Geol. 464, 14–22 (2017).
A. T. Kuiters and H. M. Sarink, “Leaching of phenolic compounds from leaf and needle litter of several deciduous and coniferous trees,” Soil Biol. Biochem. 18 (5), 475–480 (1986).
Y. Kuzyakov and E. Blagodatskaya, “Microbial hotspots and hot moments in soil: concept, review,” Soil Biol. Biochem. 83, 184–199 (2015).
C. Leyval and J. Berthelin, “Weathering of a mica by roots and rhizospheric microorganisms of pine,” Soil Sci. Soc. Am. J. 55, 1009–1016 (1991).
J. Li and R. Xu, “Adsorption of phthalic acid and salicylic acid and their effect on exchangeable Al capacity of variable-charge soils,” J. Colloid Interface Sci. 306, 3–10 (2007).
W. Ling, L. Ren, Y. Gao, X. Zhu, and B. Sun, “Impact of low-molecular-weight organic acids on the availability of phenanthrene and pyrene in soil,” Soil Biol. Biochem. 41, 2187–2195 (2009).
N. Liu, J. You, W. Shi, W. Liu, and Z. Yang, “Salicylic acid involved in the process of aluminum induced citrate exudation in Glycine max L.,” Plant Soil 352, 85–97 (2012).
L. Luo, S. Zhang, X. Q. Shan, and Y. G. Zhu, “Oxalate and root exudates enhance the desorption of p,p'-DDT from soils,” Chemosphere 63, 1273–1279 (2006).
M. J. Macielag, “Chemical properties of antimicrobials and their uniqueness,” in Antibiotic Discovery and Development, Ed. by T. Dougherty and M. Pucci (Springer-Verlag, Boston, 2012).
S. Nardi, E. Sessi, D. Pizzeghello, A. Sturaro, R. Rella, and G. Parvoli, “Biological activity of soil organic matter mobilized by root exudates,” Chemosphere 46, 1075–1081 (2002).
K. Norén and P. Persson, “Adsorption of monocarboxylates at the water/goethite interface: The importance of hydrogen bonding,” Geochim. Cosmochim. Acta 71, 5717–5730 (2007).
M. Ochs, “Influence of humified and non-humified natural organic compounds on mineral dissolution,” Chem. Geol. 132, 119–124 (1996).
E. Oburger, D. Leitner, D. L. Jones, K. C. Zygalakis, A. Schnepf, and T. Roose, “Adsorption and desorption dynamics of citric acid anions in soil,” Eur. J. Soil Sci. 62, 733–742 (2011).
F. Paris, P. Bonnaud, J. Ranger, and F. Lapeyrie, “In vitro weathering of phlogopite by ectomycorrhizal fungi. I. Effect of K and Mg deficiency on phyllosilicate evolution,” Plant Soil 177, 191–201 (1995).
F. Paris, B. Botton, and F. Lapeyrie, “In vitro weathering of phlogopite by ectomycorrhizal fungi. II. Effect of K+ and Mg2+ deficiency and N sources on accumulation of oxalate and H+,” Plant Soil 179, 141–150 (1996).
D. Pizzeghello, A. Zanella, P. Carletti, and S. Nardi, “Chemical and biological characterization of dissolved organic matter from silver fir and beech forest soils,” Chemosphere 65, 190–200 (2006).
K. Raulund-Rasmussen, O. K. Borggaard, H. C. B. Hansen, and M. Olsson, “Effect of natural organic soil solutes on weathering rates of soil minerals,” Eur. J. Soil Sci. 49 (3), 367–523 (1998).
P. R. Ryan, E. Delhaize, and D. L. Jones, “Function and mechanism of organic anion exudation from plant roots,” Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 527–560 (2001).
A. Sandnes, T. D. Eldhuset, and G. Wollebæk, “Organic acids in root exudates and soil solution of Norway spruce and silver birch,” Soil Biol. Biochem. 37, 259–269 (2005).
S. K. Song and P. M. Huang, “Dynamics of potassium release from potassium-bearing minerals as influenced by oxalic and citric acids,” Soil Sci. Soc. Am. J. 52, 383–390 (1988).
B. W. Strobel, “Influence of vegetation on low-molecular-weight carboxylic acids in soil solution—a review,” Geoderma 99, 169–198 (2001).
B. W. Strobel, C. B. Hansen, O. Borggaard, M. K. Kersen, and K. Raulund-Rasmussen, “Composition and reactivity of DOC in forest floor soil solutions in relation to tree species and soil type,” Biogeochemistry 56, 1–26 (2001).
W. Stumm, Chemistry of the Solid-Water Interface (Wiley, New York, 1992).
W. Stumm, G. Furrer, E. Wieland, and B. Zinder, “The effects of complex-forming ligands on the dissolution of oxides and alumino-silicates,” in The Chemistry of Weathering, Ed. by J. I. Drever (D. Reidel, Dordrecht, 1985), pp. 55–74.
K. Suominen, V. K. I. Tunen, and A. Smolander, “Characteristics of dissolved organic matter and phenolic compounds in forest soils under silver birch (Betula pendula), Norway spruce (Picea abies) and Scots pine (Pinus sylvestris),” Eur. J. Soil Sci. 54, 287–293 (2003).
W. Szymański, “Quantity and chemistry of water-extractable organic matter in surface horizons of Arctic soils under different types of tundra vegetation—A case study from the Fuglebergsletta coastal plain (SW Spitsbergen),” Geoderma 305, 30–39 (2017).
M. Tani and T. Higashi, “Vertical distribution of low molecular weight aliphatic carboxylic acids in some forest soils of Japan,” Eur. J. Soil Sci. 50, 217–226 (1999).
M. M. S. Tuason and J. M. Arocena, “Root organic acid exudates and properties of rhizosphere soils of white spruce (Picea glauca) and subalpine fir (Abies lasiocarpa),” Can. J. Soil Sci. 89, 287–300 (2009).
F. Valentinuzzi, S. Cesco, N. Tomasi, and T. Mimmo, “Effect of aluminium exposure on the release of organic acids and genistein from the roots of Lupinus albus L. plants,” Rhizosphere 1, 29–32 (2016).
G. F. Vance and M. B. David, “Chemical characteristics and acidity of soluble organic substances from a northern hardwood forest floor, central Maine, USA,” Geochim. Cosmochim. Acta 55, 3611–3625 (1991).
P. A. W. van Hees and U. S. Lundström, “Equilibrium models of aluminium and iron complexation with different organic acids in soil solution,” Geoderma 94, 201–221 (2000).
P. A. W. van Hees, U. S. Lundström, and R. Giesler, “Low molecular weight organic acids and their Al-complexes in soil solution—composition, distribution and seasonal variation in three podzolized soils,” Geoderma 94, 173–200 (2000).
P. A. W. van Hees, U. S. Lundström, M. Starr, and R. Giesler, “Factors influencing aluminum concentrations in soil solution from podzols,” Geoderma 94, 289–310 (2000).
P. A. W. van Hees, D. L. Jones, and D. L. Godbold, “Biodegradation of low molecular weight organic acids in coniferous forest podzolic soils,” Soil Biol. Biochem. 34, 1261–1272 (2002).
P. A. W. van Hees, U. S. Lundström, and C.‑M. Mörth, “Dissolution of microcline and labradorite in a forest O horizon extract: the effect of naturally occurring organic acids,” Chem. Geol. 189, 199–211 (2002).
P. A. W. van Hees, D. L. Godbold, G. Jentschke, and D. L. Jones, “Impact of ectomycorrhizas on the concentration and biodegradation of simple organic acids in a forest soil,” Eur. J. Soil Sci. 54, 697–706 (2003).
P. A. W. van Hees, D. L. Jones, and D. L. Godbold, “Biodegradation of low molecular weight organic acids in a limed forest soil,” Water, Air, Soil Pollut. 3, 121–144 (2003).
P. A. W. van Hees, D. L. Jones, R. Finlay, D. L. Godbold, and U. S. Lundström, “The carbon we do not see—the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: a review,” Soil Biol. Biochem. 37, 1–13 (2005).
V. Vranova, K. Rejsek, and P. Formanek, “Aliphatic, cyclic, aromatic organic acids, vitamins, carbohydrates in soil: a review,” Sci. World J. 2013, 524239 (2013). https://doi.org/10.1155/2013/524239
Y. N. Wang, M. K. Wang, S. Y. Zhuang, T. C. Tu, and K. Y. Chiang, “Characterization of low-molecular-weight organic acids and organic carbon of Taiwan red cypress, peacock pine, moso bamboo in a temperate rain forest,” Com. Soil Sci. Plant Anal. 38 (1–2), 77–91 (2007).
Y. Wang, L. Fang, L. Lin, T. Luan, and N. F. Y. Tam, “Effects of low molecular-weight organic acids and dehydrogenase activity in rhizosphere sediments of mangrove plants on phytoremediation of polycyclic aromatic hydrocarbons,” Chemosphere 99, 152–159 (2014).
W. Wang, J. Sun, C. Dong, and B. Lian, “Biotite weathering by Aspergillus niger and its potential utilisation,” J. Soils Sediments 16, 1901–1910 (2016).
C. F. Whitehead, R. F. Carbonaro, and A. T. Stone, “Adsorption of benzoic acid and related carboxylic acids onto FeOOH(goethite): the low ionic strength regime,” Aquat. Geochem. 21, 99–121 (2015).
S. A. Welch and W. J. Ullman, “The effect of organic acids on plagioclase dissolution rates and stoichiometry,” Geochim. Cosmochim. Acta 57 (12), 2725–2736 (1993).
R. K. Xu, S. C. Xiao, H. Zhang, J. Jiang, and G. L. Ji, “Adsorption of phthalic acid and Salicylic acid by two variable charge soils as influenced by sulphate and phosphate,” Eur. J. Soil Sci. 58, 335–342 (2007).
T. Yang, G. Liu, Y. Li, S. Zhu, A. Zou, J. Qi, and Y. Yang, “Rhizosphere microbial communities and organic acids secreted by aluminum-tolerant and aluminum-sensitive soybean in acid soil,” Biol. Fertil. Soils 48, 97–108 (2012).
C. Yu, J. F. Devlin, and E. Bi, “Bonding of monocarboxylic acids, monophenols and nonpolar compounds onto goethite,” Chemosphere 214, 158–167 (2019).
X. Zhang, L. Zhang, X. Zou, F. Han, Z. Yan, Z. Li, and S. Hu, “Semi-quantitative analysis of microbial production of oxalic acid by montmorillonite sorption and ATR-IR,” Appl. Clay Sci. 162, 518–523 (2018).
Funding
The work was supported by the Russian Foundation for Basic Research (project no. 19-29-05028 MK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by G. Chirikova
Rights and permissions
About this article
Cite this article
Sokolova, T.A. Low-Molecular-Weight Organic Acids in Soils: Sources, Composition, Concentrations, and Functions: A Review. Eurasian Soil Sc. 53, 580–594 (2020). https://doi.org/10.1134/S1064229320050154
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229320050154