Abstract
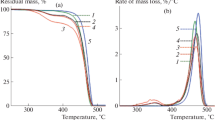
Miscibility and thermal stability of ethyl vinyl acetate (EVA) and ethylene octane (EO) copolymer blends with different compositions were investigated by thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and atomic force microscopy (AFM). The degradation behavior by TGA data under dynamic conditions in an inert atmosphere shows the blends to be immiscible. The addition of EO improves the thermal stability of EVA for all composition and temperature ranges. Using the DSC experiment, two single crystallization temperatures (T c) for the blends were obtained and the crystallization and melting enthalpy with compositions abiding by the additive rules, confirm the immiscibility of the blends. The rate of crystallization seems to be independent of blend compositions. The surface morphology using AFM shows a thin and elongated crystallites of pure EO, and a bulky and random morphology for EVA, where a perfect mixture of aforementioned structures for 50/50 blend, with the immiscible domains of both EO and EVA. The 2D-power spectral density (PSD) analysis shows the surface roughness of 50/50 blends is in between of EO and EVA. Both AFM observations and quantitative PSD results, along the line with DSC and TGA. The experimental data for miscibility and stability by TGA, DSC and AFM techniques reveal that blends of EVA/EO are immiscible in the entire range of compositions.
Similar content being viewed by others
References
R. J. Albalak, M. S. Capel, and E. L. Thomas, Polymer 39, 1647 (1998).
T. T. Wang and T. Nishi, Macromolecules 10, 421 (1977).
J. Jin, J. Du, H. Chen, and C. C. Han, Polymer 52, 6161 (2011).
F. H. Jang and E. M. Woo, Polymer 40, 2231 (1999).
C. Friedrich, C. Schwarzwalder, and R. E. Riemann, Polymer 37, 2499 (1996).
A. K. Gupta, B. K. Ratnam, and K. R. Srinivasan, J. Appl. Polym. Sci. 45, 1303 (1992).
B. Na, Q. Zhang, Q. Fu, G. Zhang, and K. Shen, Polymer 43, 7367 (2002).
A. T. Koshy, B. Kuriakose, S. Thomas, and S. Varghese, Polymer 34, 3428 (1993).
G. Takidis, D. N. Bikiaris, G. Z. Papageorgiou, D. S. Achilias, and I. Sideridou, J. Appl. Polym. Sci. 90, 841 (2003).
J. Peón, J. F. Vega, B. Del Amo, and J. Martínez-Salazar, Polymer 44, 2911 (2003).
K. A. Moly, Z. Oommen, S. S. Bhagawan, G. Groeninckx, and S. Thomas, J. Appl. Polym. Sci. 86, 3210 (2002).
T. Wu, Y. Li, D.-L. Zhang, S.-Q. Liao, and H.-M. Tan, J. Appl. Polym. Sci. 91, 905 (2004).
S. H. Goh, Thermochim. Acta 215, 291 (1993).
I. Klarić, U. Roje, and N. Stipanelov, J. Appl. Polym. Sci. 71, 833 (1999).
I. C. McNeill, J. Anal. Appl. Pyrolysis 40–41, 21 (1997).
A. Mamun, V. H. Mareau, J. Chen, and R. E. Prud’homme, Polymer 55, 2179 (2014).
A. Mamun, C. G. Bazuin, and R. E. Prud’homme, Macromolecules 48, 1412 (2015).
A. Mamun, N. Okui, and M. A. K. Khan, Can. Chem. Trans. 1, 267 (2013).
A. Mamun and N. Okui, Int. J. Eng. Res. Rev. 1, 23 (2013).
G. Aggarwal, S. J. Park, and I. Smid, Int. J. Refract. Met. Hard Mater. 24, 253 (2006).
I. Krupa, G. Miková, and A. S. Luyt, Eur. Polym. J. 43, 895 (2007).
P. Thomas-Vielma, A. Cervera, B. Levenfeld, and A. Várez, J. Eur. Ceram. Soc. 28, 763–771, (2008).
A. R. Kamdar, Y. S. Hu, P. Ansems, S. P. Chum, A. Hiltner, and E. Baer, Macromolecules 39, 1496 (2006).
R. Garcia, R. Magerle, and R. Perez, Nat. Mater. 6, 405 (2007).
W. W. Scott and B. Bhushan, Ultramicroscopy 97, 151 (2003).
T. Souier, Y. A. Samad, B. S. Lalia, R. Hashaikeh, and M. Chiesa, J. Phys. Chem. C 116, 8849 (2012).
M. P. Nikiforov, S. Gam, S. Jesse, R. J. Composto, and S. V. Kalinin, Macromolecules 43, 6724 (2010).
R. Gavrila, A. Dinescu, and D. Mardare, Roman. J. Inf. Sci. Technol. 10, 291 (2007).
M. Senthilkumar, N. K. Sahoo, S. Thakur, and R. B. Tokas, Appl. Surf. Sci. 252, 1608 (2005).
T. Itoh and N. Yamauchi, Appl. Surf. Sci. 253, 6196 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Mamun, A., Souier, M.T., Mujibur Rahman, S.M. et al. Miscibility and thermal stability of ethyl vinyl acetate and ethylene-octane copolymer blends. Polym. Sci. Ser. A 59, 397–404 (2017). https://doi.org/10.1134/S0965545X17030129
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X17030129