Abstract
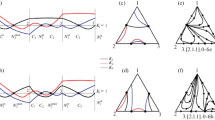
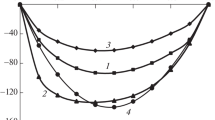
The mutual transformations of the structures of vapor–liquid equilibrium diagrams of ternary systems containing zeotropic, monoazeotropic, and biazeotropic binary components via the formation of a binary internal tangential azeotrope in the case of varying external conditions are considered. The characteristic features of the appearance of binary biazeotropy in ternary systems belonging to different types of structures of vapor–liquid equilibrium diagrams are found. The mutual transformation of vapor–liquid phase diagrams of systems of classes 3.0.0-1 and 3.[2.0.0].0-2a is illustrated via a computational experiment using an isobutyl acetate–acetic acid–sulfolane system as an example.









Similar content being viewed by others
REFERENCES
Serafimov, L.A. and Frolkova, A.K., Fundamental principle of concentration-field redistribution between separation regions as a basis for the design of technological systems, Theor. Found. Chem. Eng., 1997, vol. 31, no. 2, p. 159.
Zharov, V.T. and Serafimov, L.A., Fiziko-khimicheskie osnovy distillyatsii i rektifikatsii (Physicochemical Fundamentals of Distillation Processes), Leningrad: Khimiya, 1975.
Gaw, W.J. and Swinton, F.I., Thermodynamic properties of binary systems containing hexafluorobenzene, Trans. Faraday Soc., 1968, vol. 64, no. 8, p. 2023.
Nisel'son, L.A., Tret’yakova, K.V., and Tyurin, V.I., Crystals–liquid and vapor–liquid equilibria in the NbCl5–NbF5 and TaCl5–TaF5 systems, Zh. Neorg. Khim., 1973, vol. 18, no. 11, p. 3063.
Srivastava, R. and Smith, B.D., Total-pressure vapor-liquid equilibrium data for binary system of diethylamine with acetone, acetonitrile, and methanol, J. Chem. Eng. Data, 1985, vol. 30, no. 3, pp. 308–313.
Aucejo, A., Montón, J.B., Muñoz, R., and Wisniak, J., Double azeotropy in the benzene + hexafluorobenzene system, J. Chem. Eng. Data, 1996, vol. 41, no. 1, pp. 21–24.
Burguet, M.C., Montón, J.B., Muñoz, R., Wisniak, J., and Segura, H., Polyazeotropy in associating systems: The 2-methylpropyl ethanoate + ethanoic acid system, J. Chem. Eng. Data, 1996, vol. 41, no. 5, p. 1191.
Chai Kao, C.-P., Paulaitis, M.E., and Yokozeki, A., Double azeotropy in binary mixtures of NH3 and CHF2CF3, Fluid Phase Equilib., 1997, vol. 127, p. 191.
Shutova, G.V., Raeva, V.M., Kushner, T.M., and Serafimov, L.A., Study of biazeotropy in the propionic acid–butyl propionate system, Zh. Fiz. Khim., 1992, vol. 66, no. 3, p. 832.
Shutova, G.V., Raeva, V.M., Kushner, T.M., and Serafimov, L.A., Study of biazeotropy in the butyric acid–butyl butyrate system, Zh. Obshch. Khim., 1993, vol. 83, no. 1, p. 171.
Chai Kao, C.-P., Miller, R.N., and Sturgis, J.F., Double azeotropy in binary mixtures 1,1,1,2,3,4,4,5,5,5-decafluoropentane + tetrahydrofuran, J. Chem. Eng. Data, 2001, vol. 46, p. 229.
Nisel'son, L.A. and Astakhova, G.V., Liquid–vapor equilibrium in the sulfur–phosphorus system with three extremal points, Dokl. Akad. Nauk SSSR, 1970, vol. 192, no. 6, p. 1391.
Serafimov, L.A, Chelyuskina, T.V., and Sharonova, E.A., Biazeotropy in three-phase systems, Vestn. MITHT, 2010, vol. 5, no. 5, p. 52.
Serafimov, L.A., Thermodynamic and topological analysis of heterogeneous equilibrium diagrams of multicomponent mixtures, Russ. J. Phys. Chem. A, 2002, vol. 76, no. 8, p. 1211.
Serafimov, L.A. and Chelyuskina, T.V., Principles of classifying diagrams for different types of biazeotropic ternary mixtures, Russ. J. Phys. Chem. A, 2011, vol. 85, no. 5, p. 767.
Serafimov, L.A., Chelyuskina, T.V., and Yakushev, R.A., Thermodynamic and topological analysis of the formation of internal tangential azeotropes in binary two-phase systems, Tonkie Khim. Tekhnol., 2015, vol. 10, no. 4, p. 41.
Zhang, C., Wan, H., Xue, L., and Guan, G., Investigation on isobaric vapor liquid equilibrium for acetic acid + water + (n-propyl acetate or iso-butyl acetate), Fluid Phase Equilib., 2011, vol. 305, pp. 68–75.
Chelyuskina, T.V., Mityushkina, I.A., Chernyshova, M.A., and Frolkova, A.K., Mathematical modeling of the evolution of biazeotropy in the isobutyl acetate– acetic acid system, Vestn. MITHT, 2011, vol. 6, no. 4, p. 47.
Chelyuskina, T.V., Mityushkina, I.A., Frolkova, A.K., and Chernyshova, M.A., Separation of biazeotropic mixtures with the use of highly volatile additional substances, Khim. Tekhnol., 2011, vol. 12, no. 12, p. 730.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Serafimov, L.A., Chelyuskina, T.V., Polkovnichenko, A.V. et al. The Analysis of the Mutual Transformations of the Structures of Diagrams of Ternary Systems via the Formation of Binary Internal Tangential Azeotropes. Theor Found Chem Eng 52, 963–974 (2018). https://doi.org/10.1134/S0040579518040401
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579518040401