Abstract
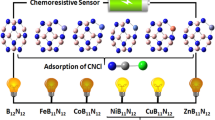
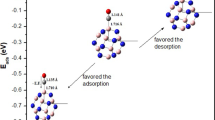
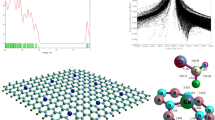
The electronic properties and adsorption behavior of cyanogen chloride (CNCl) on pure and metal-doped (Mg, Al, and Ge) B12N12 nanoclusters is studied by the density functional theory (DFT) calculations. We presented the adsorption behavior of CNCl from their N-side and Cl-side upon the pure and metal-doped B12N12 nanocluster calculated using the Perdew–Burke–Ernzerhof (PBE) functional and 6‑311++G** basis set. The important parameters required to confirm the stable nature and electronic structure of B12N12 nanocluster are calculated with the assistance of binding energy and the change of energy gap (∆Eg). The total density of states (TDOS) plot is constructed for B12N12 nanocluster (isolated and with adsorbed CNCl molecule), which confirms the transfer of electrons proceeds between the B12N12 and the CNCl. Additionally, the contrast is made between the electron density of isolated and CNCl adsorbed B12N12 nanocluster to enunciate the application of B11GeN12 nanocluster as a gas sensor for detecting CNCl.




Similar content being viewed by others
REFERENCES
D. W. Kononen, Bull. Environ. Contam. Toxicol. 41, 371 (1988).
X. Yang and C. Shang, Water Res. 39, 1709 (2005).
Z. Xiao, L. B. Kong, X. Li, S. Yu, X. Li, Y. Jiang, Z. Yao, S. Ye, C. Wang, T. Zhang, K. Zhou, and S. Li, Sens. Actuators, B 274, 235 (2018).
S. W. Thomas, K. Venkatesan, P. Müller, and T. M. Swager, J. Am. Chem. Soc. 128, 16641 (2006).
E. L. Cochran, F. J. Adrian, and V. A. Bowers, J. Chem. Phys. 36, 1938 (1962).
B. Cancho, F. Ventura, and M. T. Galceran, J. Chromatogr. A 897, 307 (2000).
A. Shokuhi Rad and K. Ayub, Int. J. Hydrogen Energy 41, 22182 (2016).
D. Golberg, Y. Bando, Y. Huang, T. Terao, M. Mitome, C. Tang, and C. Zhi, ACS Nano 4, 2979 (2010).
M. Fu, H. Xing, X. Chen, R. Zhao, C. Zhi, and C. Wu, Anal. Bioanal. Chem. 406, 5751 (2014).
K. Dhungana and R. Pati, Sensors 14, 17655 (2014).
E. Tazikeh-Lemeski, A. Soltani, M. Taghi Baei, M. Bezi Javan, and S. Moazen Rad, Adsorption 24, 585 (2018).
A. Soltani and M. Bezi Javan, RSC Adv. 5, 90621 (2015).
A. Soltani, M. T. Baei, E. Tazikeh Lemeski, and A. A. Pahlevani, Superlatt. Microstruct. 75, 716 (2014).
A. Soltani, M. T. Baei, M. Mirarab, M. Sheikhi, and E. Tazikeh Lemeski, J. Phys. Chem. Solids 75, 1099 (2014).
A. Soltani, M. T. Baei, M. Ramezani Taghartapeh, E. Tazikeh Lemeski, and S. Shojaee, Struct. Chem. 26, 685 (2015).
M. T. Baei, S. Z. Sayyed-Alangi, A. Soltani, M. Bahari, and A. Masoodi, Monatsh. Chem. 142, 1 (2011).
A. Soltani, M. T. Baei, E. Tazikeh Lemeski, and M. Shahini, Superlatt. Microstruct. 76, 315 (2014).
M. Bezi Javan, A. Soltani, A. S. Ghasemi, E. Tazikeh Lemeski, N. Gholamic, and H. Balakheyli, Appl. Surf. Sci. 411, 1 (2017).
M. T. Baei, A. S. Ghasemi, E. Tazikeh Lemeski, A. Soltani, and N. Gholami, J. Clust. Sci. 27, 1081 (2016).
A. Soltani, M. T. Baei, A. S. Ghasemi, E. Tazikeh Lemeski, and Komail Hosseni Amirabadi, Superlatt. Microstruct. 75, 564 (2014).
M. T. Baei, M. Ramezani Taghartapeh, A. Soltani, K. Hosseni Amirabadi, and N. Gholami, Russ. J. Phys. Chem. B 11, 354 (2017).
A. Soltani, A. Sousaraei, M. Mirarab, and H. Balakheyli, J. Saudi Chem. Soc. 21, 270 (2017).
P. Pakravan and S. A. Siadati, J. Mol. Graph. Model. 75, 80 (2017).
A. Soltani, A. Sousaraei, M. Bezi Javan, M. Eskandaric, and H. Balakheyli, New J. Chem. 40, 7018 (2016).
M. Bezi Javan, A. Soltani, Z. Azmoodeh, N. Abdolahi, and N. Gholami, RSC Adv. 6, 104513 (2016).
A. Soltani, M. Ramezani Taghartapeh, V. Erfani-Moghadam, M. Bezi Javane, F. Heidari, M. Aghaei, and P. J. Mahon, Mater. Sci. Eng. C 92, 216 (2018).
N. Abdolahi, M. Aghaei, A. Soltani, Z. Azmoodeh, H. Balakheyli, and F. Heidari, Spectrochim. Acta A 204, 348 (2018).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, et al., Gaussian 09, Revision A.01 (Gaussian, Inc., Wallingford, CT, 2009).
A. Soltani, M. Ramezani Taghartapeh, M. Bezi Javan, P. J. Mahon, Z. Azmoodeh, E. Tazikeh Lemeski, and I. V. Kityk, Phys. E (Amsterdam, Neth.) 97, 239 (2018).
E. Tazikeh Lemeski, A. Soltani, M. Taghi Baei, M. Bezi Javan, and S. Moazen Rad, Adsorption 24, 585 (2018).
A. Soltani, M. Bezi Javan, M. S. Hoseininezhad-Namin, N. Tajabor, E. Tazikeh Lemeski, and F. Pourarian, Synth. Met. 234, 1 (2017).
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery, J. Comput. Chem. 14, 1347 (1993).
V. Nagarajan and R. Chandiramouli, Comput. Theor. Chem. 1150, 63 (2019).
R. Bhuvaneswari, V. Nagarajan, and R. Chandiramouli, J. Mol. Graph. Model. 89, 13 (2019).
Morteza Rouhani, J. Mol. Struct. 1181, 518 (2019).
E. Vessally, R. Moladust, S. M. Mousavi-Khoshdel, M. D. Esrafili, A. Hosseinian, and L. Edjlali, Thin Solid Films 645, 363 (2018).
M. T. Baei, M. R. Taghartapeh, A. Soltani, K. Hosseni Amirabadi, and N. Gholami, Russ. J. Phys. Chem. B 11, 354 (2017).
A. Soltani, A. Sousaraei, M. Mirarab, and H. Balakheyli, J. Saudi Chem. Soc. 21, 270 (2017).
A. Soltani, M. T. Baei, A. S. Ghasemi, E. Tazikeh Lemeski, and K. Hosseni Amirabadi, Superlatt. Microstruct. 75, 564 (2014).
L. Shiyan, J. Xingqiang, and Z. Guoying, Chem. Phys. Lett. 726, 77 (2019).
H. Ahmoum, M. Boughrara, and M. Kerouad, Superlatt. Microstruct. 127, 186 (2019).
M. D. Esrafili, F. Mohammadian-Sabet, and P. Nematollahi, Int. J. Hydrogen Energy 41, 20172 (2016).
S. Larki, E. Shakerzadeh, E. Chigo Anota, and R. Behjatmanesh-Ardakani, Chem. Phys. 526, 1104 (2019).
E. Shakerzadeh, E. Khodayar, and S. Noorizadeh, Comput. Mater. Sci. 118, 155 (2016).
A. Bahrami, S. Seidi, T. Baheri, and M. Aghamohammadi, Superlatt. Microstruct. 64, 265 (2013).
E. Vessally, M. D. Esrafili, R. Nurazar, P. Nematollahi, and A. Bekhradnia, Struct. Chem. 28, 735 (2017).
S. K. Gupta, H. He, I. Lukacevic, and R. Pandey, Phys. Chem. Chem. Phys. 19, 30370 (2017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatemeh Azimi, Elham Tazikeh-Lemeski Exploring Adsorption Behavior of Cyanogen Chloride Molecule on Boron Nitride Nanocluster from First-Principles. Russ. J. Phys. Chem. 94, 2141–2147 (2020). https://doi.org/10.1134/S0036024420100039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420100039