Abstract
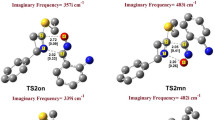
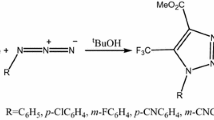
Copper-catalyzed azide–alkyne cycloaddition (CuAAC) is a straightforward way for making covalent connections between building blocks containing various functional groups. It is widely used in organic synthesis, medicinal chemistry, polymer chemistry, and bioconjugation applications. Using copper acetonitrile as catalyst for click reactions (CuAAC) lead to a non-concerted reaction, and affect Parr functions indices to determine the polar sites, therefore predict the favorable regioisomer (1,4-regioisomer) and explain the contradiction obtained to the experimental results. The huge difference of activation barriers between catalyzed and uncatalyzed reaction indicate that is a selective reaction.





Similar content being viewed by others
REFERENCES
R. Huisgen, Pure Appl. Chem. 61, 613 (1989).
R. Huisgen, G. Szeimies, and L. Moebius, Chem. Ber. 100, 2494 (1967).
C. W. Tornøe and M. Meldal, in Peptides 2001, Proceedings of the American Peptide Symposium (Am. Peptide Soc., Kluwer Academic, San Diego, 2001), p. 263.
C. W. Tornøe, C. Christensen, and M. Meldal, J. Org. Chem. 67, 3057 (2002).
V. V. Rostovtsev, L. G. Green, V. V. Fokin, and B. K. Sharpless, Angew. Chem., Int. Ed. 41, 2596 (2002).
L. Wolff, Ann. 394, 23 (1912); Ann. 394, 59 (1912); Ann. 394, 68 (1912).
W. Lwowski, in 1, 3- Dipolar Cycloaddition Chemistry, Ed. by A. Padwa (Wiley-Interscience, New York, 1984), Vol. 1, Chap. 5.
M. M. Majireck and S. M. Weinreb, J. Org. Chem. 71, 8680 (2006).
I. Fleming, Frontiers Orbitals and Organic Chemical Reactions (Wiley, London, 1976).
R. G. Parr and R. G. Pearson, J. Am. Chem. Coc. 105, 7512 (1983).
I. Marhraoui, E. M. El Hadrami, A. Ben-Tama, and M. El Asri, J. Marocain Chim. Heterocycl. 9, 59 (2010).
A. D. Becke, Phys. Rev. A 38, 3098 (1988).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B 37, 785 (1988).
A. K. Nacereddine, W. Yahia, S. Bouacha, and A. Djerourou, Tetrahedron Lett. 51, 2617 (2010).
R. G. Parr, L. von Szentpaly, and S. Liu, J. Am. Chem. Soc. 121, 1922 (1999).
R. G. Parr and R. G. Pearson, J. Am. Chem. Soc. 105, 7512 (1983);
R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules (Oxford Univ. Press, New York, 1989).
L. R. Domingo, P. Pérez, and J. Sáez, RSC Adv. 3, 1486 (2013).
E. Chamorro, P. Pérez, and L. R. Domingo, Chem. Phys. Lett. 582, 141 (2013).
L. R. Domingo, E. Chamorro, and P. Pérez, J. Org. Chem. 73, 4615 (2008);
L. R. Domingo and P. Pérez, Org. Biomol. Chem. 9, 7168 (2011).
W. Kohn and L. Sham, J. Phys. Rev. 140, 1133 (1965).
L. R. Domingo, E. Chamorro, and P. Perez, J. Org. Chem. 73, 4615 (2008);
P. Jaramillo, L. R. Domingo, E. Chamorro, and P. Perez, J. Mol. Struct.: THEOCHEM 865, 68 (2008).
L. R. Domingo, M. Jose Aurell, P. Perez, and R. Contreras, J. Phys. Chem. A 106, 6871 (2002).
M. Jose Aurell, R. Luis Domingo, P. Perez, and R. Contreras, Tetrahedron 60, 11503 (2004).
F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless, and V. V. Fokin, J. Am. Chem. Soc. 127, 210 2005.
E. J. Yoo, M. Ahlquist, I. Bae, K. B. Sharpless, V. V. Fokin, and S. Chang, J. Org. Chem. 73, 5520 (2008).
ACKNOWLEDGMENTS
We are grateful to the “Association Marocaine des Chimistes Théoriciens” (AMCT) for its pertinent help concerning the programs.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Adib Ghaleb, Aouidate, A., Lakhlifi, T. et al. Theoretical Study of Copper Acetonitrile Effects on Parr Functions Indices and Regioselectivity Using Density Functional Theory (DFT). Russ. J. Phys. Chem. 92, 2464–2471 (2018). https://doi.org/10.1134/S0036024418120038
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418120038