Abstract
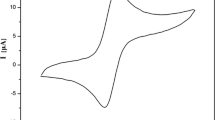
The work is devoted to the theoretical and experimental study of the electrochemical characteristics of the closo-decaborate anion. The regions of electrochemical transformations of the [B10H10]2– anion for non-aqueous solvents of different structures (acetonitrile, dichloromethane, and tetrahydrofuran) have been studied by the method of cyclic voltammetry. It has been shown that, under the conditions of anodic oxidation, a dimerization product, the [B20H18]2– anion, is formed. For the synthesis of octadecahydroeicosaborate anion, electrochemical synthesis has been used for the first time.








Similar content being viewed by others
REFERENCES
M. Yan, Y. Kawamata, and P. S. Baran, Chem. Rev. 117, 13230 (2017). https://doi.org/10.1021/acs.chemrev.7b00397
Ghiasi Reza, M. Rahimi, and P. R. Jamaat, Russ. J. Inorg. Chem. 65, 69 (2020). https://doi.org/10.1134/S0036023620010088
A. X. Tian, Y. B. Fu, H. P. Ni, et al., Russ. J. Inorg. Chem. 65, 359 (2020). https://doi.org/10.1134/S0036023620030183
Ming Zhao and Xiaoli Zhou, Russ. J. Inorg. Chem. 65, 1166 (2020). https://doi.org/10.1134/s0036023620080203
X.-W. Liu, N.-Y. Liu, Y.-Q. Deng, et al., Russ. J. Inorg. Chem. 65, 1186 (2020). https://doi.org/10.1134/s0036023620080094
A. V. Sonone, M. Shaikh, M. Farooqui, et al., Russ. J. Inorg. Chem. 65, 390 (2020). https://doi.org/10.1134/S003602362003016X
I. N. Klyukin, A. S. Novikov, A. P. Zhdanov, et al., Mendeleev Commun. 30, 88 (2020). https://doi.org/10.1016/j.mencom.2020.01.029
M. Keener, C. Hunt, T. G. Carroll, et al., Nature 577, 652 (2020). https://doi.org/10.1038/s41586-019-1926-4
V. V. Avdeeva, E. A. Malinina, A. V. Vologzhanina, et al., Inorg. Chim. Acta 509, 119693 (2020). https://doi.org/10.1016/j.ica.2020.119693
S. P. Fisher, A. W. Tomich, S. O. Lovera, et al., Chem. Rev. 119, 8262 (2019). https://doi.org/10.1021/acs.chemrev.8b00551
A. V. Shmal’ko and I. B. Sivaev, Russ. J. Inorg. Chem. 64, 1726 (2019). https://doi.org/10.1134/S0036023619140067
C. Nandi, S. Kar, M. Zafar, et al., Inorg. Chem. 59, 3537 (2020). https://doi.org/10.1021/acs.inorgchem.0c00122
H. Yan, D. Tu, J. Poater, et al., Angew. Chem., Int. Ed. Engl. (2020). https://doi.org/10.1002/anie.201915290
M. H. Hsu, C. Y. Hsieh, M. Kapoor, et al., Bioorg. Chem. 98, 103729 (2020). https://doi.org/10.1016/j.bioorg.2020.103729
D. Olid, R. Nunez, C. Vinas, et al., Chem. Soc. Rev. 42, 3318 (2013). https://doi.org/10.1039/c2cs35441a
I. Viñas and C. Teixidor, Future Med. Chem. 5, 617 (2013). https://doi.org/10.4155/fmc.13.41
I. B. Sivaev and V. V. Bregadze, Eur. J. Inorg. Chem., No. 11, 1433 (2009). https://doi.org/10.1002/ejic.200900003
I. B. Sivaev, A. V. Prikaznov, and D. Naoufal, Collect. Czechoslov. Chem. Commun. 75, 1149 (2010). https://doi.org/10.1135/cccc2010054
K. Y. Zhizhin, A. P. Zhdanov, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 55, 2089 (2010). https://doi.org/10.1134/S0036023610140019
A. A. Semioshkin, I. B. Sivaev, and V. I. Bregadze, Dalton Trans. 11, 977 (2008). https://doi.org/10.1039/b715363e
Z. Laila, O. Yazbeck, F. A. Ghaida, et al., J. Organomet. Chem. 910, 121132 (2020). https://doi.org/10.1016/j.jorganchem.2020.121132
A. P. Zhdanov, V. V. Voinova, I. N. Klyukin, et al., J. Clust. Sci. 2 (2019). https://doi.org/10.1007/s10876-019-01628-2
V. K. Burianova, D. S. Bolotin, A. S. Mikherdov, et al., New J. Chem. 42, 8693 (2018). https://doi.org/10.1039/c8nj01018h
A. V. Nelyubin, I. N. Klyukin, A. P. Zhdanov, et al., Russ. J. Inorg. Chem. 64, 1750 (2019). https://doi.org/10.1134/S0036023619140043
A. P. Zhdanov, A. V. Nelyubin, I. N. Klyukin, et al., Russ. J. Inorg. Chem. 64, 841 (2019). https://doi.org/10.1134/S0036023619070180
V. V. Avdeeva, M. I. Buzin, A. O. Dmitrienko, et al., Chem. A Eur. J. 23, 16819 (2017). https://doi.org/10.1002/chem.201703285
M. F. Hawthorne, K. Shelly, and F. Li, Chem. Commun. 2, 547 (2002). https://doi.org/10.1039/b110076a
E. Bernhardt, D. J. Brauer, M. Finze, et al., Angew. Chem., Int. Ed. Engl. 46, 2927 (2007). https://doi.org/10.1002/anie.200604077
E. L. Muetterties, Inorg. Chem. 231, 1450 (1964).
R. L. Middaugh and F. J. Farha, Inorg. Chem., 4147 (1965).
A. P. Schmitt and R. L. Middaugh, Inorg. Chem. 13, 163 (1974). https://doi.org/10.1021/ic50131a031
D. B. G. Williams and M. Lawton, Org. Chem. 75, 8351 (2010). https://doi.org/10.1021/jo101589h
M. F. Hawthorne, R. L. Pilling, and W. H. Knoth, Inorg. Synth., 16 (1967). https://doi.org/10.1002/9780470132401.ch6
F. Neese, WIREs Comput. Mol. Sci. 2, 73 (2012). https://doi.org/10.1002/wcms.81
T. Lu and F. Chen, J. Comput. Chem. 33, 580 (2011). https://doi.org/10.1002/jcc.22885
E. D. Glendening and J. K. Badenhoop, et al., NBO 7.0 (Theor. Chem. Inst., Univ. of Wisconsin, Madison, 2018).
V. V. Avdeeva, E. A. Malinina, L. V. Goeva, et al., Dokl. Chem. 474, 141 (2017). https://doi.org/10.1134/S0012500817060052
ACKNOWLEDGMENTS
The research was carried out using the equipment of the Center for Collective Use of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, functioning within the framework of the State Assignment of the Kurnakov Institute in the field of fundamental scientific research.
Funding
The study was supported by the Russian Foundation for Basic Research (project nos. 19-29-08047 and 20-33-70217).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Voinova, V.V., Klyukin, I.N., Novikov, A.S. et al. Electrochemical Properties of the closo-Decaborate Anion [B10H10]2– and a New Method for Preparation of the [B20H18]2– Anion. Russ. J. Inorg. Chem. 66, 295–304 (2021). https://doi.org/10.1134/S0036023621030190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621030190