Abstract
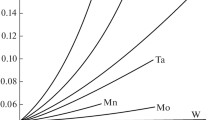
Based on the CALPHAD method, a thermodynamic description of the Fe–M–V–NB–Ti–C–N system (where M is Al, Cr, Mn, Ni, or Si) has been constructed and, using this description, the solubilities of carbonitrides in austenite for low-alloy low-carbon steels with V, Nb, and Ti have been calculated using 10G2FB steel as an example. The influence of the alloy composition and temperature on the composition and amount of carbonitride phases and on the concentration of these elements in the solid solution has been analyzed.
Similar content being viewed by others
References
V. V. Popov and I. I. Gorbachev, “Analysis of solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: I. Description of thermodynamic properties. Computation procedure,” Phys. Met. Metallogr. 98, 344–354 (2004).
Certificate of State Registration of Computer Program 2011618874 (IMP Equilibrium, November 15, 2011).
V. V. Popov and I. I. Gorbachev, “Analysis of solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: II. Solubility of carbides, nitrides, and carbonitrides in the Fe–V–C, Fe–V–N, and Fe–V–C–N systems,” Phys. Met. Metallogr. 99, 286–299 (2005).
I. I. Gorbachev and V. V. Popov, “Analysis of solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: III. Solubility of carbides, nitrides, and carbonitrides in the Fe–Ti–C, Fe–Ti–N, and Fe–Ti–C–N systems,” Phys. Met. Metallogr. 108, 484–495 (2009).
I. I. Gorbachev and V. V. Popov, “Analysis of solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: IV. Solubility of carbides, nitrides, and carbonitrides in the Fe–Nb–C, Fe–Nb–N, and Fe–Nb–C–N systems,” Phys. Met. Metallogr. 110, 52–61 (2010).
I. I. Gorbachev and V. V. Popov, “Thermodynamic simulation of the Fe–V–Nb–C–N system using the CALPHAD method,” Phys. Met. Metallogr. 111, 495–502 (2011).
I. I. Gorbachev, V. V. Popov, and A. Yu. Pasynkov, “Thermodynamic simulation of the formation of carbonitrides in steels with Nb and Ti,” Phys. Met. Metallogr. 113, 687–695 (2012).
I. I. Gorbachev, V. V. Popov, and A. Yu. Pasynkov, “Thermodynamic simulation of the formation of carbonitrides in steels with V and Ti,” Phys. Met. Metallogr. 113, 974–981 (2012).
I. I. Gorbachev, V. V. Popov, and A. Yu. Pasynkov, “Thermodynamic calculations of carbonitride formation in low–alloy low–carbon steels containing V, Nb, and Ti,” Phys. Met. Metallogr. 115, 69–76 (2014).
I. I. Gorbachev, V. V. Popov, and A. Yu. Pasynkov, “Simulation of precipitate ensemble evolution in steels with V and Nb,” Phys. Met. Metallogr. 116, 356–366 (2015).
I. I. Gorbachev, A. Yu. Pasynkov, and V. V. Popov, “Prediction of the austenite-grain size of microalloyed steels based on the simulation of the evolution of carbonitride precipitates,” Phys. Met. Metallogr. 116, 1127–1192 (2015).
H. L. Lukas, S. G. Fries, and B. Sundman, Computational Thermodynamics: The CALPHAD Method (Cambridge Univ., Cambridge, 2007).
M. Hillert and L.-I. Staffonsson, “The regular solution model for stoichiometric phases and ionic melts,” Acta Chem. Scand. 24, 3618–3626 (1970).
B. Sundman and J. Agren, “A regular solution model for phase with several components and sublattices, suitable for computer applications,” J. Phys. Chem. Solids 42, 297–301 (1981).
M. Hillert and M. Jarl, “Model for alloying effects in ferromagnetic metals,” CALPHAD 2, 227–238 (1978).
G. Inden, “Determination of chemical and magnetic interexchange energies in bcc alloys. III. Application to ferromagnetic alloys,” Z. Metallkd. 68, 529–534 (1977).
H. Ohtani, M. Yamano, and M. Hasebe, “Thermodynamic analysis of the Fe–Al–C ternary system by incorporating ab initio energetic calculations into the CALPHAD approach,” Iron Steel Inst. Jpn. Int. 44, 1738–1747 (2004).
D. Connetable, J. Lacazea, P. Maugisb, and B. Sundman, “A CALPHAD assessment of Al–C–Fe system with the κ carbide modelled as an ordered form of the fcc phase,” CALPHAD 32, 361–370 (2008).
B. Sundman, I. Ohnuma, N. Dupin, U. R. Kattner, and S. G. Fries, “An assessment of the entire Al–Fe system including D03 ordering,” Acta Mater. 57, 2896–2908 (2009).
M. Hillert and S. Jonsson, “An assessment of the Al–Fe–N system,” Metallur. Trans. A 23, 3141–3149 (1992).
K. Frisk, “A thermodynamic evaluation of the Cr–N, Fe–N, Mo–N and Cr–Mo–N systems,” CALPHAD 15, 79–106 (1991).
L. S. Darken, R. P. Smith, and E. W. Filer, “Solubility of gaseous nitrogen in gamma iron and the effect of alloying constituents–aluminum nitride precipitation,” Trans. AIME 191, 1174–1179 (1951).
J. Krueger, H. D. Kunze, and E. Schuermann, in Gases and Carbon in Metals, Ed. by E. Fromm and E. Gebhardt, (Springer-Verlag, Berlin, 1976), pp. 578–613.
V. T. Witusiewicz, B. Hallstedt, A. A. Bondar, U. Hecht, S. V. Sleptsov, and T. Ya. Velikanova, “Thermodynamic description of the Al–C–Ti system,” J. Alloys Compd. 623, 480–496 (2015).
V. T. Witusiewicz, A. A. Bondar, U. Hecht, S. Rex, and T. Ya. Velikanova, “The Al–B–Nb–Ti system. III. Thermodynamic re-evaluation of the constituent binary system Al–Ti,” J. Alloys Comp. 465, 64–77 (2008).
L. F. S. Dumitrescu, M. Hillert, and B. A. Sundman, “Reassessment of Ti–C–N based on a critical-review of available assessments of Ti–N and Ti–C,” Z. Metallkd. 90, 534–541 (1999).
G. Chen and B. Sundman, “Thermodynamic analysis of the Ti–Al–N system,” J. Phase Equil. 19, 146–160 (1998).
J. Gao, Ch. Li, N. Wang, and Zh. Du, “Thermodynamic analysis of the Ti–Al–N system,” J. Beijing Univ. Sci. Technol. 15, 420–424 (2008).
S. Jonsson, “Assessment of the Ti–N system,” Z. Metallkd. 87, 691–702 (1996).
P. J. Spencer, “The decisive role of calorimetric measurements in equilibrium phase diagram calculations,” J. Therm. Anal. 41, 1305–1318 (1994).
V. T. Witusiewicz, A. A. Bondar, U. Hecht, and T. Ya. Velikanova, “The Al–B–Nb–Ti system. IV. Experimental study and thermodynamic re-evaluation of the binary Al–Nb and ternary Al–Nb–Ti systems,” J. Alloys Compd. 472, 133–161 (2009).
N. Saunders, “System Al–V,” in COST-507 Project: Thermochemical Database for Light Metal Alloys (Vol. 2), Ed. by A. Ansara, T. Dinsdale, and M. H. Rand (Office for Official Publications of the European Communities, Luxembourg, 1998), pp. 95–98.
M. Hillert and C. Qiu, “A thermodynamic assessment of the Fe–Cr–Ni–C system,” Metall. Trans. A 22, 2187–2198 (1991).
B.-J. Lee, “On the stability of Cr carbides,” CALPHAD 16, 124–149 (1992).
J.-O. Andersson, “A thermodynamic evaluation of the Fe–Cr–C system,” Metall. Trans. A 19, 627–636 (1988).
L. Kjellqvist and M. Selleby, “Adding C to the thermodynamic description of the Cr–Fe–Ni–O system,” CALPHAD 33, 393–397 (2009).
J. Bratberg and K. Frisk, “An experimental and theoretical analysis of the phase equilibria in the Fe–Cr–V–C system,” Metall. Mater. Trans. A 35, 3649–3663 (2004).
A. Khvan, B. Hallstedt, and C. Broeckmann, “A thermodynamic evaluation of the Fe–Cr–C system,” CALPHAD 46, 24–33 (2014).
K. Frisk, “A thermodynamic evaluation of the Cr–Fe–N system,” Metall. Trans. A 21, 2477–2488 (1990).
N. Saunders, “System Cr–Ti,” in COST-507 Project: Thermochemical Database for Light Metal Alloys (Version 2.1) (Vol. 2),–Ed. by A. Ansara, T. Dinsdale, and M. H. Rand (Office for Official Publications of the European Communities, Luxembourg, 1999/2003).
B.-J. Lee and D. N. Lee, “A thermodynamic evaluation of the Fe–Cr–V–C system,” J. Phase Equil. 13, 349–364 (1992).
A. Khvan, B. Hallstedt, and K. Chang, “Thermodynamic assessment of Cr–Nb–C and Mn–Nb–C systems,” CALPHAD 39, 54–61 (2012).
B.-J. Lee, “A thermodynamic evaluation of the Fe–Cr–Mn–C system,” CALPHAD 24, 1017–1025 (1993).
W. Huang, “Thermodynamic properties of the Fe–Mn–V–C system,” Metall. Mater. Trans. A 22, 1911–1920 (1991).
A. V. Khvan and B. Hallstedt, “Thermodynamic description of the Fe–Mn–Nb–C system,” CALPHAD 39, 62–69 (2012).
W. Huang, “An assessment of the Fe–Mn system,” CALPHAD 13, 243–252 (1989).
D. Djurovic, B. Hallstedt, J. von Appen, and R. Dronskowski, “Thermodynamic assessment of the Mn–C system,” CALPHAD 34, 279–285 (2010).
D. Djurovic, B. Hallstedt, J. von Appen, and R. Dronskowski, “Thermodynamic assessment of the Fe–Mn–C system,” CALPHAD 35, 479–491 (2011).
S. Liu, B. Hallstedt, D. Music, and Y. Du, “Ab initio calculations and thermodynamic modeling for the Fe-Mn–Nb system,” CALPHAD 38, 43–58 (2012).
A. V. Khvan and B. Hallstedt, “Thermodynamic assessment of Fe–Mn–Nb–N and Nb–C–N systems,” CALPHAD 40, 10–15 (2013).
B.-J. Lee, “Thermodynamic assessment of the Fe-Nb–Ti–C–N system,” Metall. Mater. Trans. A 32, 2423–2439 (2001).
C. Qiu and A. F. Guillermet, “Predictive approach to the entropy of manganese nitrides and calculation of the Mn–N phase diagram,” Z. Metallkd. 84, 11–22 (1993).
C. Qiu, “A thermodynamic evaluation of the Fe–Mn–N system,” Metall. Trans. A 24, 629–645 (1992).
A. Gabriel, P. Gustafson, and I. Ansara, “A thermodynamic evaluation of the C–Fe–Ni system,” CALPHAD 11, 203–218 (1987).
A. T. Dinsdale, “SGTE data for pure elements,” CALPHAD 15, 317–425 (1991).
B.-J. Lee, “Revision of thermodynamic descriptions of the Fe–Cr and Fe–Ni liquid phases,” CALPHAD 17, 251–268 (1993).
M. Mathon, D. Connetable, B. Sundman, and J. Lacaze, “CALPHAD-type assessment of the Fe–Nb–Ni ternary system,” CALPHAD 33, 136–161 (2009).
K. Santhy and K. C. H. Kumar, “Thermodynamic reassessment of Nb–Ni–Ti system with order–disorder model,” J. Alloys Compd. 619, 733–747 (2015).
H. Chen and Y. Du, “Refinement of the thermodynamic modeling of the Nb–Ni system,” CALPHAD 30, 308–315 (2006).
K. C. H. Kumar, P. Wollants, and L. Delaey, “Thermodynamic calculation of Nb–Ti–V phase diagram,” CALPHAD 18, 71–79 (1994).
N. Saunders, “System Ni–V,” in COST-507 Project: Thermochemical Database for Light Metal Alloys (Vol. 2), Ed. by A. Ansara, T. Dinsdale, and M. H. Rand (Office for Official Publications of the European Communities, Luxembourg, 1998), pp. 261–263.
J. Lacaze and B. Sundman, “An assessment of the Fe–C–Si system,” Metall. Mater. Trans. A 22, 2211–2223 (1991).
J. Miettinen, “Reassessed thermodynamic solution phase data for ternary Fe–Si–C system,” CALPHAD 22, 231–256 (1998).
X. Ma, Ch. Li, and W. Zhang, “The thermodynamic assessment of the Ti–Si–N system and the interfacial reaction analysis,” J. Alloys Compd. 394, 138–147 (2005).
H. Liang and Y. A. Chang, “Thermodynamic modeling of the Nb–Si–Ti ternary system,” Intermetallics 7, 561–570 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.I. Gorbachev, V.V. Popov, A.Yu. Pasynkov, 2016, published in Fizika Metallov i Metallovedenie, 2016, Vol. 117, No. 12, pp. 1277–1287.
Rights and permissions
About this article
Cite this article
Gorbachev, I.I., Popov, V.V. & Pasynkov, A.Y. Calculations of the influence of alloying elements (Al, Cr, Mn, Ni, Si) on the Solubility of carbonitrides in low-carbon low-alloy steels. Phys. Metals Metallogr. 117, 1226–1236 (2016). https://doi.org/10.1134/S0031918X16120061
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031918X16120061