Abstract
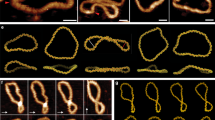
Supercoiled 3993-bp pGEMEX DNA immobilized on four substrates (freshly cleaved mica, standard amino mica, and modified amino mica with an increased or decreased surface charge density in comparison to standard amino mica) has been visualized by atomic force microscopy in the air. Plectonomically supercoiled DNA molecules, as well as single molecules with an extremely high compaction level (i.e., with a significantly higher superhelix density compared to those previously observed experimentally or estimated theoretically), have been visualized on modified amino mica with an increased surface charge density. The distance between nucleotide pairs along the duplex axis has been determined by measuring the contour length of individual oversupercoiled DNA molecules. The estimated rise per base pair varies from 1.94 to 2.19 Å. These supercoiled DNA molecules, which are compressed like a spring and have a decreased rise per base pair compared to previously known DNA forms are considered to be a new form of DNA, S-DNA. A model of S-DNA has been constructed. Molecules of S-DNA may be an intermediate in the course of the compaction of single supercoiled DNA molecules into spheroids and minitoroids. The DNA oversupercoiling, followed by the compression of the supercoiled molecules, has been shown to be accounted for by a high surface charge density of amino mica on which DNA molecules are immobilized.
Similar content being viewed by others
References
Ivanov V. 1983. DNA double helix. Mol. Biol. 17, 616–621.
Saenger W. 1984. Principles of Nucleic Acid Structure. N.Y.: Springer.
Wang J. 1979. Helical repeat of DNA in solution. Proc. Natl. Acad. Sci. USA. 76, 200–203.
Cluzel P., Lebrun A., Heller C., Lavery R., Viovy J., Chatenay D., Caron F. 1996. DNA: An extensible molecule. Science. 271, 792–794.
Smith S., Cui Y., Bustamante C. 1996. Overstretching B-DNA: The elastic response of individual double-stranded and single-stranded DNA molecules. Science. 271, 795–799.
Leuba S., Karymov M., Tomschik M., Ramjit R., Smith P., Zlatanova J. 2003. Assembly of single chromatin fibers depends on the tension in the DNA molecule: Magnetic tweezers study. Proc. Natl. Acad. Sci. USA. 100, 495–500.
Bennink M., Leuba S., Leno G., Zlatanova J., De Grooth B., Greve J. 2001. Unfolding individual nucleosomes by stretching single chromatin fibers with optical tweezers. Nature Struct. Biol. 8, 606–610.
Rivetti C., Codeluppi S., Dieci G., Bustamante C. 2003. Vizualizing RNA extrusion and DNA wrapping in transcription elongation complexes of bacteriae and eukaryotic RNA polymerases. J. Mol. Biol. 326, 1413–1426.
Rivetti C., Codeluppi S. 2001. Accurate length determination of DNA molecules visualized by atomic force microscopy: Evidence for a partial B-to A-form transition on mica. Ultramicroscopy. 87, 55–66.
Lyubchenko Y., Gall A., Shlyakhtenko L., Harrington R., Jacobs B., Oden P., Lindsay S. 1992. Atomic force microscopy imaging of double stranded DNA and RNA. J. Biomol. Struct. Dyn. 10, 589–606.
Lyubchenko Y., Shlyakhtenko L. 1997. Visualization of supercoiled DNA with atomic force microscopy in situ. Proc. Natl. Acad. Sci. USA. 94, 496–501.
Shlyahtenko L., Gall A., Filonov A., Cerovac Z., Lushnikov A., Lyubchenko Y. 2003. Silatrane-based surface chemistry for immobilization of DNA, protein-DNA complexes and other biological materials. Ultramicroscopy. 97, 279–287.
Limansky A. 2001. Analysis of aminomodified mica as a substrate for atomic force microscopy of nucleic acids. Biopolim. Kletka. 17, 292–297.
Limansky A., Shlyakhtenko L., Schaus S., Henderson E., Lyubchenko Y. 2002. Aminomodified probes for atomic force microscopy. Probe Microsc. 2, 227–234.
Butt H. 1991. Measuring electrostatic, van der Waals, and hydration forces in electrolyte solutions with an atomic force microscope. Biophys. J. 60, 1438–1444.
Hansma H., Golan R., Hsieh W., Daubendiek S., Kool E. 1999. Polymerase activities and RNA structures in the atomic force microscope. J. Struct. Biol. 127, 240–247.
Cherny D., Jovin T. 2001. Electron and scanning force microscopy studies of alterations in supercoiled DNA tertiary structure. J. Mol. Biol. 313, 295–307.
Tanigawa M., Okada T. 1998. Atomic force microscopy of supercoiled DNA structure on mica. Anal. Chim. Acta. 365, 19–25.
Bussiek M., Mucke N., Langowski J. 2003. Polylysine-coated mica can be used to observe systematic changes in the supercoiled DNA conformation by scanning force microscopy in solution. Nucleic Acids Res. 31, 1–10.
Limansky A. 2002. Analysis of aminomodified probes for atomic force microscopy of biological molecules. Biopolim. Kletka. 18, 62–70.
Limansky A., Limanskaya O. 2002. Analysis of genomic DNA by atomic force microscopy. Tsitol. Genet. 36 6–30.
Mazur G., Jernigan R., Sarai A. 2003. Conformational effects upon DNA stretching. Mol. Biol. 37, 277–287.
Shlyakhtenko L., Gall A., Weimer J., Hawn D., Lyubchenko Y. 1999. Atomic force microscopy imaging of DNA covalently immobilized on a functionalized mica substrate. Biophys. J. 77, 568–576.
Vologodsky A. 1988. Topologiya I fizicheskie svoistva kol’tsevykh DNK (Topology and Properties of Circular DNAs). Moscow: Nauka.
Boles T., White, Cozzarelli N. 1990. Structure of plectonomically supercoiled DNA. J. Mol. Biol. 213, 931–951.
Conwell C., Vilfan I., Hud N. 2003. Controlling the size of nanoscale toroidal DNA condensates with static curvature and ionic strength. Proc. Natl. Acad. Sci. USA. 100, 9296–9301.
Kemura K., Rybenkov V., Crisona N., Hirano T., Cozzarelli N. 1999. 13S Condensin actively reconfigures DNA by introducing global positive writhe: Implications for chromosome condensation. Cell. 98, 239–248.
Hizume K., Yoshimura S., Maruyama H., Kim J., Wada H., Takeyasu K. 2002. Chromatin reconstitution: Development of a salt-dialysis method monitored by nanotechnology. Arch. Histol. Cytol. 65, 405–413.
Yoshimura S., Hizume K., Murakami A., Sutani T., Takeyasu K., Yanagida M. 2002. Condensin architecture and interaction with DNA: Regulatory non-SMC subunits bind to the head of SMC heterodimer. Curr. Biol. 12, 508–513.
Polyanichko A.M., Chikhirzhina E.V., Andrushchenko V.V., Kostyleva E.I., Wieser H., Vorob’ev V.I. 2004. The effect of Ca2+ ions on DNA compaction in the complex with nonhistone chromosomal HMG1 protein. Mol. Biol. 38, 701–712.
Limansky A. 2003. Atomic force microscopy: From visualizing DNA and protein molecules to measuring the force of intermolecular interactions. Usp. Sovrem. Biol. 123, 531–542.
Newlin D., Miller K., Pilch D. 1984. Interactions of molecules with nucleic acids: 7. Intercalation and T-A specificity of daunomycin in DNA. Biopolymers. 23, 139–158.
Author information
Authors and Affiliations
Additional information
Original Russian Text © L.A. Limanskaya, A.P. Limansky, 2006, published in Molekulyarnaya Biologiya, 2006, Vol. 40, No. 1, pp. 122–136.
Rights and permissions
About this article
Cite this article
Limanskaya, L.A., Limansky, A.P. S-DNA, over-supercoiled DNA with a 1.94-to 2.19-Å rise per base pair. Mol Biol 40, 107–120 (2006). https://doi.org/10.1134/S0026893306010158
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0026893306010158