Abstract—
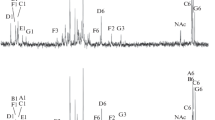
Lipopolysaccharide was isolated by phenol extraction from the surface membrane of the nitrogen-fixing endophytic rhizobacterium Herbaspirillum seropedicae, strain Z78. The lipopolysaccharide’s lipid A contained 3-hydroxydecanoic, 3-hydroxydodecanoic, dodecanoic, tetradecanoic, and hexadecanoic acids. The 3-hydroxydodecanoic acid was amide-linked to the sugar backbone of the lipid A. The structure of the O-polysaccharide from H. seropedicae Z78 was established for the first time. It is characterized by heterogeneity and by the presence of glycerol, a component rarely found in gram-negative bacteria. The O polysaccharide of H. seropedicae Z78 was found to consist of two types of repeating units: one represented by glycerol-1-phosphate and the other by the glycerol-1-phosphate of the backbone, which is substituted at the 2-position by N-acetyl-D-glucosamine. The lipopolysaccharide of the H. seropedicae Z78 was weakly toxic to warm-blooded animals and moderately and dose-dependently induced interleukin synthesis by human whole blood cells and NO synthesis by mouse splenocytes. This may indicate that the H. seropedicae lipopolysaccharide is a promising antagonist of classical endotoxins.




Similar content being viewed by others
REFERENCES
Baldani, V.L.D., Baldani, J.I., Olivares, F.L., and Döbereiner, J., Identification and ecology of Herbaspirillum seropedicae and closely related Pseudomonas rubrisubalbicans, Symbiosis, 1992, vol. 13, pp. 65−73.
Balsanelli, E., Tuleski, T.R., de Baura, V.A., Yates, M.G., Chubatsu, L.S., Pedrosa, F.O., de Souza, E.M., and Monteiro, R.A., Maize root lectins mediate the interaction with Herbaspirillum seropedicae via N-acetyl glucosamine residues of lipopolysaccharides, PLoS One, 2013, vol. 8. e77001. doi 10.1371/journal.pone.0077001
Berg, G., Eberl, L., and Hartmann, A., The rhizosphere as a reservoir for opportunistic human pathogenic bacteria, Environ. Microbiol., 2005, vol. 7, pp. 1673–1685.
Bock, K. and Pedersen, C., Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides, Adv. Carbohydr. Chem. Biochem., 1983, vol. 41, pp. 27–66.
Conrad, H.E., Methylation of carbohydrates with methylsulfinyl anion and methyl iodide in dimethyl sulfoxide, Methods Carbohydr. Chem., 1972, vol. 6, pp. 361–364.
Green, L.C., Wagner, D.A., Glogowski, J. Skipper, P.L., Wishnok, J.S., and Tannenbaum, S.R., Analysis of nitrate, nitrite, and (15 N) nitrate in biological fluids, Anal. Biochem., 1982, vol. 126, pp. 131–138.
Hitchcock, P.J. and Brown, T.M., Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels, J. Bacteriol., 1983, vol. 154, pp. 269−277.
Komaniecka, I., Zdzisinska, B., Kandefer-Szerszen, M., and Choma, A., Low endotoxic activity of lipopolysaccharides isolated from Bradyrhizobium, Mesorhizobium, and Azospirillum strains, Microbiol. Immunol., 2010, vol. 54, pp. 717−725.
Kondakova, A.N., Fudala, R., Senchenkova, S.N., Shashkov, A.S., Knirel, Yu.A., and Kaca, W., Structure of a lactic acid ether-containing and glycerol phosphate-containing O-polysaccharide from Proteus mirabilis O40, Carbohydr. Res., 2005, vol. 340, pp. 1612–1617.
Konnova, S.A., Makarov, O.E., Skvortsov, I.M., and Ignatov, V.V., Isolation, fractionation and some properties of polysaccharides produced in a bound form by Azospirillum brasilense and their possible involvement in Azospirillum-wheat root interactions, FEMS Microbiol. Lett., 1994, vol. 118, pp. 93–99.
Kul’shin, V.A., Yakovlev, A.P., Avaeva, S.N., and Dmitriev, B.A., Improved method for lipopolysaccharide isolation from gram-negative bacteria, Mol. Genet. Mikrobiol. Virusol., 1987, no. 5, pp. 44−46.
Mayer, H., Tharanathan, R.N., and Weckesser, J., Analysis of lipopolysaccharides of Gram-negative bacteria, Methods Microbiol., 1985, vol. 18, pp. 157–207.
Monteiro, R.A., Balsanelli, E., Tuleski, T., Faoro, H., Cruz, L.M., Wassem, R., de Baura, V.A., Tadra-Sfeir, M.Z., Weiss, V., DaRocha, W.D., Muller-Santos, M., Chubatsu, L.S., Huergo, L.F., Pedrosa, F.O., and de Souza, E.M., Genomic comparison of the endophyte Herbaspirillum seropedicae SmR1 and the phytopathogen Herbaspirillum rubrisubalbicans M1 by suppressive subtractive hybridization and partial genome sequencing, FEMS Microbiol. Ecol., 2012, vol. 80, pp. 441–451.
Naumova, I.B., Shashkov, A.S., Tul’skaya, E.M., Streshinskaya, G.M., Kozlova, Y.I., Potekhina, N.V., Evtushenko, L.I., and Stackebrandt, E., Cell wall teichoic acids: structural diversity, species specificity in the genus Nocardiopsis, and chemotaxonomic perspective, FEMS Microbiol. Rev. 2001, vol. 25, pp. 269–284.
Pedraza, R.O., Recent advances in nitrogen-fixing acetic acid bacteria, Int. J. Food Microbiol., 2008, vol. 125, pp. 25–35.
Perepelov, A.V., Liu, B., Senchenkova, S.N., Shashkov, A.S., Feng, L., Wang, L., and Knirel, Yu.A., Structure of O-antigen and functional characterization of O-antigen gene cluster of Salmonella enterica O47 containing ribitol phosphate and 2-acetimidoylamino-2,6-dideoxy-L-galactose, Biochemistry (Moscow), 2009, vol. 74, pp. 416–420.
Prozorovskii, V.B., Prozorovskaya, M.P., and Devchenko, V.M., Express method for determination of the average effective dose and its erroe, Farmakol. Toksikol., 1978, no. 4, pp. 497–502.
Reinhold-Hurek, B. and Hurek, T., Life in grasses: diazotrophic endophytes, Trends Microbiol., 1998, vol. 139, pp. 139−144.
Sawardeker, J.S., Sloneker, J.H., and Jeanes, A. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography, Anal. Chem., 1965, vol. 37, pp. 1602–1604.
Schletter, J., Heine, H., Ulmer, A.J., and Rietschel, E.T., Molecular mechanisms of endotoxin activity, Arch. Microbiol., 1995, vol. 164, pp. 383–389.
Serrato, R.V., Balsanelli, E., Sassaki, G.L., Carlson, R.W., Muszynski, A., Monteiro, R.A., Pedrosa, F.O., Souza, E.M., and Iacomini, M., Structural analysis of Herbaspirillum seropedicae lipid A and of two mutants defective to colonize maize roots, Int. J. Biol. Macromol., 2012, vol. 51, pp. 384–391.
Serrato, R.V., Lipopolysaccharides in diazotrophic bacteria, Front. Cell Infect. Microbiol., 2014, vol. 4. 119. doi 10.3389/fcimb.2014.00119
Serrato, R.V., Sassaki, G.L., Cruz, L.M., Carlson, R.W., Muszynski, A., Monteiro, R.A., Pedrosa, F.O., Souza, E.M., and Iacomoni, M., Chemical composition of lipopolysaccharides isolated from various endophytic nitrogen-fixing bacteria of the genus Herbaspirillum, Can. J. Microbiol., 2010, vol. 56, pp. 342−347.
Shashkov, A.S., Wang, M., Turdymuratov, E.M., Hu, Sh., Arbatsky, N.P., Guo, X., Wang, L., and Knirel, Yu.A., Structural and genetic relationships of closely related O‑antigens of Cronobacter spp. and Escherichia coli: C. sakazakii G2594 (serotype O4)/E. coli O103 and C. malonaticus G3864 (serotype O1)/E. coli O29, Carbohydr. Res., 2015, vol. 404, pp. 124−131.
Smol’kina, O.N., Shishonkova, N.S., Yurasov, N.A., and Ignatov, V.V., Capsular and extracellular polysaccharides of the diazotrophic rhizobacterium Herbaspirillum seropedicae Z78, Microbiology (Moscow), 2012, vol. 81, pp. 317–323.
Tsai, C.M. and Frasch, C.E., A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels, Anal. Biochem., 1982, vol. 119, pp. 115−119.
Varbanets, L.D., Structure and biological role of Mycobacterium, Corynebacterium, and Nocardia polysaccharides, Mikrobiol. Zh., 1988, vol. 50, no. 5, pp. 98–107.
Wu, C.H., Chen, T.L., Chen, T.G., Ho, W.P., Chiu, W.T., and Chen, R.M., Nitric oxide modulates pro- and anti-inflammatory cytokines in lipopolysaccharide-activated macrophages, J. Trauma, 2003, vol. 55, pp. 540–545.
Zdorovenko, E.L., Varbanets, L.D., Brovarskaya, O.S., Valueva, O.A., Shashkov, A.S., and Knirel’, Yu.A., Lipopolysaccharide of Budvicia aquatic 97U124: immunochemical properties and structure, Microbiology (Moscow), 2011, vol. 80, pp. 372–377.
Zhao, L., Ohtaki, Yu., Yamaguchi, K., Matsushita, M., Fujita, T., Yokochi, T., Takada, H., and Endo, Ya., LPS-induced platelet response and rapid shock in mice: contribution of O-antigen region of LPS and involvement of the lectin pathway of the complement system, Blood, 2002, vol. 100, pp. 3233–3239.
Zych, K., Toukach, F.P., Arbatsky, N.P., Kolodziejska, K., Senchenkova, S.N., Shashkov, A.S., Knirel, Yu.A., and Sidorczyk, Z., Structure of the O-specific polysaccharide of Proteus mirabilis D52 and typing of this strain to Proteus serogroup O33, Eur. J. Biochem., 2001, vol. 268, pp. 4346–4351.
Wu, C.H., Chen, T.L., Chen, T.G., Ho, W.P., Chiu, W.T., and Chen, R.M., Nitric oxide modulates pro- and anti-inflammatory cytokines in lipopolysaccharide-activated macrophages, J. Trauma, 2003, vol. 55, pp. 540–545.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Dedyukhina
Rights and permissions
About this article
Cite this article
Velichko, N.S., Surkina, A.K., Fedonenko, Y.P. et al. Structural Peculiarities and Biological Properties of the Lipopolysaccharide from Herbaspirillum seropedicae Z78. Microbiology 87, 635–641 (2018). https://doi.org/10.1134/S002626171805017X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002626171805017X