Abstract
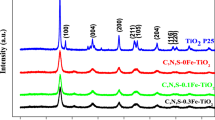
Undoped, single-doped, and codoped TiO2 nanoparticles were prepared by the sol-gel method and characterized with X-ray diffraction (XRD), the Brunauer-Emmett-Teller (BET)-specific surface area (SBET), UV-Vis absorption spectra (UV-Vis), and X-ray photoelectron spectroscopy (XPS). Their photocatalytic activity was evaluated by methyl orange (MO) degradation in an aqueous suspension under UV or simulated solar light illumination. XRD showed that all samples calcined at 600°C preserved the anatase structure, and doping inhibited the increase of crystallite size. The BET result revealed that doping improved the surface area of TiO2. UV-Vis indicated that Fe3+-doping broadened the absorption profile of TiO2. XPS demonstrated that doping was advantageous to absorb more surface hydroxyl groups or chemisorbed water molecules. Photocatalytic degradation showed that the photocatalytic activity of TiO2 codoped with Fe3+ and Ho3+ ions was markedly improved. This was ascribed to the fact that there was a cooperative action in the two doped elements. Fe3+-doping broadens the absorption profile, improves photo utilization of TiO2, and then generates more electronhole pairs. Ho3+-doping restrains the increase in grain size and retards the recombination of photo-generated electrons and holes.
Similar content being viewed by others
References
Lachheb, H., Puzenat, E., Houas, A., Ksibi, M., Elaloui, E., Guillard, C., and Herrmann J.M., Appl. Catal., B, 2002, vol. 39, p. 75.
Zielinska, B., Grzechulska, J., and Morawski, A.W., J. Photochem. Photobiol., A, 2003, vol. 157, p. 65.
Vionea, D., Minero, C., Maurinoa, V., Carlotti, M.E., Picatonotto, T., and Pelizzetti, E., Appl. Catal., B, 2005, vol. 58, p. 79.
Bessekhouad, Y., Robert, D., and Weber, J.V., J. Photochem. Photobiol., A, 2004, vol. 163, p. 569.
Wang, R., Xin, J.H., Yang, Y., Liu, H., Xu, L., and Hu, J., Appl. Surf. Sci., 2004, vol. 227, p. 312.
Sakthivel, S., Neppolian, B., Shankar, M.V., Arabindoo, B., Palanichamy, M., and Murugesan, V., Sol. Energy Mater. Sol. Cells, 2003, vol. 11, p. 65.
Topalov, A.S., Sojic, D.V., Molnar-Gabor, D.A., Abramovic, B.F., and Comor, M.I., Appl. Catal., B, 2004, vol. 54, p. 125.
Zhang, L., Kanki, T., Sano, N., and Toyoda, A., Sep. Purif. Technol., 2003, vol. 31, p. 105.
Wu, J.C.S. and Chen, C.H., J. Photochem. Photobiol., A, 2004, vol. 163, p. 509.
Yang, Y., Li, X., Chen, J., and Wang, L., J. Photochem. Photobiol., A, 2004, vol. 163, p. 517.
Wang, C., Bottcher, C., Bahnemann, D.W., and Dohrmann, J.K., J. Nanopart. Res., 2004, vol. 6, p. 119.
Sun, B., Reddy, E.P., and Smirniotis, P.G., Appl. Catal., B, 2005, vol. 57, p. 139.
Zhang, W., Li, Y., Zhu, S., and Wang, F., Catal. Today, 2004, vols. 93–95, p. 589.
Xu, J., Lu, M., Guo, X., and Li, H., J. Mol. Catal. A: Chem., 2005, vol. 226, p. 123.
Xie, Y. and Yuan, C., Mater. Res. Bull., 2004, vol. 39, p. 533.
Xie, Y. and Yuan, C., Appl. Catal., B, 2003, vol. 46, p. 251.
Baiju, K.V., Sibu, C.P., Rajesh, K., Krishna, Pillai P., Mukundan, P., Warrier, K.G.K., and Wunderlich, W., Mater. Chem. Phys., 2005, vol. 90, p. 123.
Xu, A.W., Gao, Y., and Liu, H.Q., J. Catal., 2002, vol. 207, p. 151.
Chen, S.Y., Ting, C.C., and Hsieh, W.F., Thin Solid Films, 2003, vol. 434, p. 171.
Wei, H., Wu, Y., Lun, N., and Zhao, F., J. Mater. Sci., 2004, vol. 39, p. 1305.
Yang, P., Mengkai, XuD., Yuan, D., Song, C., Liu, S., and Cheng, X., Opt. Mater., 2003, vol. 24, p. 497.
Wang, J., Yin, S., Komatsu, M., and Sato, T., J. Eur. Ceram. Soc., 2005, vol. 25, p. 3207.
Zhang, Z., Wang, C., Zakaria, R., and Ying, J.Y., J. Phys. Chem. B, 1998, vol. 102, p. 10 871.
Wang, C.Y., Bottcher, C., Bahnemann, D.W., and Dohrmann, J.K., J. Mater. Chem., 2003, vol. 13, p. 2322.
Wang, Y.Q., Cheng, H.M., Zhang, L., Hao, Y.Z., Ma, J.M., Xu, B., and Li, W.H., J. Mol. Catal. A: Chem., 2000, vol. 151, p. 205.
Gou, Y.Q., Chen, D.Y., and Su, Z.X., Appl. Catal., A, 2004, vol. 261, p. 15.
Liu, F.M. and Wang, T.M., Appl. Surf. Sci., 2002, vol. 195, p. 284.
Zhang, W., Li, Y., Zhu, S., and Wang, F., Chem. Phys. Lett., 2003, vol. 373, p. 333.
Borgmann, D., Hums, E., Hopfengartner, G., Wedler, G., Spitznagel, G.W., and Rademacher, I., J. Electron. Spectrosc. Relat. Phenom., 1993, vol. 63, p. 91.
Atrens, A. and Lim, A.S., Appl. Phys. A, 1990, vol. 51, p. 411.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Kinetika i Kataliz, 2008, Vol. 49, No. 2, pp. 293–299.
This article was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Shi, J., Zheng, J., Hu, Y. et al. Photocatalytic degradation of organic compounds in aqueous systems by Fe and Ho codoped TiO2 . Kinet Catal 49, 279–284 (2008). https://doi.org/10.1134/S002315840802016X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002315840802016X