Abstract
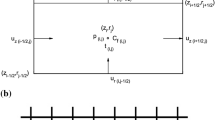
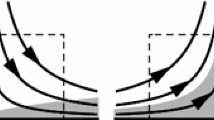
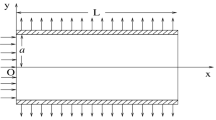
The interendothelial cleft is the major transport pathway across the endothelium for hydrophilic solutes including albumin and low density lipoprotein. Previous models of arterial wall transport have assumed that the entire endothelial cell surface is available for transport from the fluid (blood) phase. One of the consequences of a cleft-mediated solute uptake mechanism is the limited area available for mass transport. This effect, together with the influence of a predominantly longitudinal cleft orientation in relation to flow, dramatically alters the fluid–phase mass transport characteristics relative to what has been assumed previously in analyzing vascular solute uptake problems. We have used a finite element computational model to simulate fluid phase transport to a longitudinal endothelial cleft under realistic wall shear rate conditions. Our numerical results show reduced dependence of the mass transfer rate on the wall shear rate compared to the classical Leveque solution for mass transport in a cross-flow configuration and confirm the significance of the wall and not the fluid as the limiting resistance to transport of macromolecules. © 2002 Biomedical Engineering Society.
PAC2002: 8719Rr, 8716Uv, 8716Dg, 8710+e, 8715Vv
Similar content being viewed by others
REFERENCES
Ackerberg, R. C., R. D. Patel, and S. K. Gupta. The heat/mass transfer to a finite strip at small Peclet numbers. J. Fluid Mech. 86:49-65, 1978.
Adamson, R. H. Microvascular endothelial cell shape and size in situ. Microvasc. Res. 46:77-88, 1993.
Adamson, R. H., and C. C. Michel. Pathways through the intercellular clefts of frog mesenteric capillaries. J. Physiol. (London) 466:303-327, 1993.
Basmadjian, D. The effect of flow and mass transport in thrombogenesis. Ann. Biomed. Eng. 18:685-709, 1990.
Caro, C. G., and R. M. Nerem. Transport of 14C-4-cholesterol between serum and wall in the perfused dog common carotid artery. Circ. Res. 32:187-205, 1973.
Chuang, P. T., H. J. Cheng, S. J. Lin, K. M. Jan, M. M. L. Lee, and S. Chien. Macromolecular transport across arterial and venous endothelium in rats. Arteriosclerosis (Dallas) 10:188-197, 1990.
Fu, B., F. E. Curry, R. H. Adamson, and S. Weinbaum. A model for interpreting the tracer labeling of interendothelial clefts. Ann. Biomed. Eng. 25:375-379, 1997.
Huang, Y., D. Rumschitzki, S. Chien, and S. Weinbaum. A fiber matrix model for the growth of macromolecular leakage spots in the arterial intima. J. Biomech. Eng. 116:430-445, 1994.
Huang, Z. J., and J. M. Tarbell. Numerical simulation of mass transfer in porous media of blood vessel walls. Am. J. Physiol. Heart Circ. Physiol. 273:H464-H477, 1997.
Juhasz, N. M., and W. M. Deen. Mass transfer in a tube with wall flux confined to evenly spaced discrete areas. Chem. Eng. Sci. 48:1745-1752, 1993.
Leveque, M. A. Le problem de phechange de chaleur a l'interieur d'un tube cylindrique. Ann. Mines 13:256-299, 1928.
Lin, S. J., K. M. Jan, and S. Chien. Role of dying endothelial cells in transendothelial macromolecular transport. Arteriosclerosis (Dallas) 10:703-709, 1990.
Lin, S. J., K. M. Jan, S. Weinbaum, and S. Chien. Transendothelial transport of low density lipoprotein in association with cell mitosis in rat aorta. Arteriosclerosis (Dallas) 9:230-236, 1989.
Marcus, B. C., C. W. Wyble, K. L. Hynes, and B. L. Gewertz. Cytokine-induced increases in endothelial permeability occur after adhesion molecule expression. Surgery (St. Louis) 120:411-417, 1996.
Milton, S. G., and V. P. Knutson. Comparison of the function of the tight junctions of endothelial cells and epithelial cells in regulating the movement of electrolytes and macromolecules across the cell monolayer. J. Cell Physiol. 144:498-504, 1990.
Noria, S., D. B. Cowan, A. I. Gotlieb, and B. L. Langille. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ. Res. 85:504-514, 1999.
Phillips, C. G., K. H. Parker, and W. Wang. A model for flow through discontinuities in the tight junction of the endothelial intercellular cleft. Bull. Math. Biol. 56:723-741, 1994.
Qiu, Y., and J. M. Tarbell. Numerical simulation of oxygen mass transfer in a compliant curved tube model of a coronary artery. Ann. Biomed. Eng. 28:26-38, 2000.
Saito, Y. A theoretical study on the diffusion current at the stationary electrodes of circular and narrow band types. Rev. Polarography 15:177-187, 1968.
Secomb, T. W., R. Hsu, and A. R. Pries. A model for red blood cell motion in glycocalyx-lined capillaries. Am. J. Physiol. Heart Circ. Physiol. 274:H1016-H1022, 1998.
Sill, H., Y. S. Chang, J. R. Artman, J. A. Frangos, T. M. Hollis, and J. M. Tarbell. Shear stress increases hydraulic conductivity of cultured endothelial monolayers. Am. J. Physiol. Heart Circ. Physiol. 268:H535-H543, 1995.
Staddon, J. M., K. Herrenknecht, C. Smales, and L. L. Rubin. Evidence that tyrosine phosphorylation may increase tight junction permeability. J. Cell. Sci. 108:609-619, 1995.
Tarbell, J. M., and Y. Qiu. Arterial wall mass transport: The possible role of blood phase resistance in the localization of arterial disease. In: The Biomedical Engineering Handbook, 2nd ed., edited by J. D. Bronzino. New York: CRC, 2000, pp. 100.1-100.15.
Tedgui, A., and M. J. Lever. Filtration through damaged and undamaged rabbit thoracic aorta. Am. J. Physiol. Heart Circ. Physiol. 247:H784-H791, 1984.
Truskey, G. A., W. L. Roberts, R. A. Herrmann, and R. A. Malinauskas. Measurement of endothelial permeability to 125I-low density lipoproteins in rabbit arteries by use if en face preparations. Circ. Res. 71:883-897, 1992.
Vink, H., and B. R. Duling. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ. Res. 79:581-589, 1996.
Vink, H., and B. R. Duling. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am. J. Physiol. Heart Circ. Physiol. 278:H285-H289, 2000.
Wada, S., and T. Karino. Theoretical study on flow-dependent concentration polarization of low density lipoproteins at the luminal surface of a straight artery. Biorheology 36:207-223, 1999.
Weinbaum, S., G. Tzeghai, P. Ganatos, R. Pfeffer, and S. Chien. Effects of cell turnover and leaky junctions on arterial macromolecular transport. Am. J. Physiol. Heart Circ. Physiol. 248:H945-H960, 1985.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hodgson, L., Tarbell, J.M. Solute Transport to the Endothelial Intercellular Cleft: The Effect of Wall Shear Stress. Annals of Biomedical Engineering 30, 936–945 (2002). https://doi.org/10.1114/1.1507846
Issue Date:
DOI: https://doi.org/10.1114/1.1507846