-
PDF
- Split View
-
Views
-
Cite
Cite
J. Ramón De Lucas, Oscar Martínez, Patricia Pérez, M. Isabel López, Susana Valenciano, Fernando Laborda, The Aspergillus nidulans carnitine carrier encoded by the acuH gene is exclusively located in the mitochondria, FEMS Microbiology Letters, Volume 201, Issue 2, July 2001, Pages 193–198, https://doi.org/10.1111/j.1574-6968.2001.tb10756.x
Close - Share Icon Share
Abstract
The location of the Aspergillus nidulans carnitine/acyl-carnitine carrier (ACUH) was studied. ACUH with a His-tag at its N-terminus was over-expressed in Escherichia coli and purified by Ni2+ affinity chromatography. The purified protein was utilised to raise polyclonal antibodies which were characterised by Western blotting. For localisation studies A. nidulans T1 strain, that contains the acuH gene under control of the strong promoter alcAp, was derived. Results obtained demonstrate the exclusively mitochondrial localisation of ACUH and therefore exclude the targeting of the acuH gene product to the peroxisomal membrane.
1 Introduction
Cloning and characterisation of the acuH gene demonstrated the identification of the carnitine/acyl-carnitine carrier of the fungus Aspergillus nidulans, the first homologue of the human carnitine/acyl-carnitine carrier identified in eukaryotic microorganisms [1].
In humans the deficiency of the carnitine/acyl-carnitine translocase, encoded by the cact gene [2], causes the most severe disorder of fatty acid β-oxidation and can be lethal within a few hours after birth [2;3;4]. Moreover, lethality associated with this deficiency has also been shown in Drosophila melanogaster and in the nematode Caenorhabditis elegans [5,6]. By contrast, in the fungus A. nidulans the acuH gene is only essential for growth on long-chain fatty acids such as oleate and C2 compounds such as acetate and not for the utilisation of alternative carbon sources such carbohydrates [1,7]. This important advantage highlights this aerobic microorganism as a great model to analyse important aspects of this genetic and metabolic disease.
In lower and higher eukaryotic cells the existence of two carnitine/acyl-carnitine carriers, one located in the mitochondrial inner membrane and the other in the peroxisomal membrane, has been proposed [1,8,9]. The function of the mitochondrial carnitine carrier is the transport of acyl-carnitines into the mitochondrial matrix [10], while the peroxisomal carnitine carrier should be involved in the transport of acyl-carnitines from the peroxisomes to the cytosol [1,8,9].
Since no candidate gene encoding the peroxisomal carnitine carrier has been identified in any eukaryotic organism yet, it was of interest to determine whether the A. nidulans acuH gene encodes only the mitochondrial carnitine carrier or both the mitochondrial and peroxisomal carnitine carrier. The hypothetical dual localisation of the acuH gene product would indicate the existence of a new carrier belonging to the mitochondrial carrier family but also located in the peroxisomal membrane [11,12] and would resemble the case of the related microbial enzymes mitochondrial and peroxisomal carnitine acetyltransferases which are encoded by a single gene [13,14].
The aim of this work has been to determine the subcellular localisation of the acuH gene product in A. nidulans. The A. nidulans carnitine/acyl-carnitine carrier (ACUH) protein was over-expressed in Escherichia coli with a N-terminal histidine tail and purified by affinity chromatography. Polyclonal antibodies raised against ACUH were used to localise the protein within the fungal cells by electron immunocytochemistry.
2 Materials and methods
2.1 Bacterial strains and plasmids
E. coli DH5α (BRL) was utilised for plasmid amplification. E. coli BL21 (DE3) (Novagen) was employed for expression of the His-tagged ACUH.
Plasmid pGEMTeasy (Promega, USA) was used to subclone PCR-amplified fragments. Plasmid pD1 [15], kindly donated by Dr. Miguel Peñalva (Centro de Investigaciones Biológicas, CSIC Madrid, Spain), was employed to construct pD1ACUH encoding the His-tagged ACUH. The vector pGroESL [16] was also utilised for over-expression of ACUH in E. coli. Plasmid pAL3 containing the Neurospora crassa pyr-4 gene as a fungal selectable marker and the A. nidulans alcA promoter (alcAp) [17] was employed to construct the fungal expression vector pALACUH.
2.2 A. nidulans strains and growth conditions
A. nidulans R21 (pabaA1 yA2) is the wild-type strain used in this work. This strain was incubated on 1% glucose minimal medium [18] (repressing conditions) on a shaker (200 rpm) at 37°C for 24 h. The mycelia obtained were washed with sterile water and shifted to 6 mM oleate minimal medium (inducing conditions) for 12 h.
A. nidulans G-191 (pabaA1 pyrG89, uaY9 fwA1) was used in transformation experiments with pALACUH. The T1 transformant was selected and incubated, as described above, on 1% glucose minimal medium (repressing conditions) for 24 h and shifted to minimal medium with 0.1 M threonine+6 mM oleate (inducing conditions) for 12 h.
Induced mycelia of strains R21 and T1 were used to isolate the mitochondrial and peroxisomal (17 000×g) enriched fractions as described elsewhere [19]. Moreover, induced mycelia of both strains were processed for immunoelectron microscopy.
2.3 Construction of the bacterial expression vector
A cDNA fragment containing all the acuH coding sequence except the first nine codons was amplified by PCR using the oleate-induced cDNA library earlier described [1]. Primers ACUHBAM 5′-CCGTCCAGGAGGATCCGTCAAGGCTGCC-3′ and ACUHECO 5′-GGTACTAGAATTCTTAGTCGAAAAGCTTGGC-3′ introducing a BamHI site and an EcoRI site respectively were used in this PCR reaction. The PCR-amplified fragment was subcloned into pGEMTeasy. The BamHI and EcoRI insert encoding amino acids 10–326 of ACUH was ligated into the expression vector pD1 [15] previously digested with BamHI and EcoRI. The generated vector pD1ACUH encodes an ACUH recombinant protein in which its first nine amino acids have been replaced by the peptide MGH10SSGHIDDDDKHMGS.
2.4 Bacterial expression and purification of ACUH
E. coli BL21 (DE3) cells harbouring plasmids pD1ACUH and pGroESL were grown in LB medium (50 μg ampicillin ml−1, 34 μg chloramphenicol ml−1) at 37°C until OD600 reached 0.7. Synthesis of ACUH was then induced for 3 h by addition of IPTG (100 μM) while shaking (200 rpm) at 37°C.
Induced cells were collected by centrifugation (7 000×g, 10 min, 4°C) and washed with binding buffer (50 mM imidazole, 500 mM NaCl, 20 mM Tris–HCl pH 7.9). Cells were lysed in a French Press (1000 PSI) and after centrifugation (15 000×g, 15 min, 4°C) the pellet containing the His-tagged ACUH protein was retained. Solubilisation of the recombinant protein was achieved by adding urea to 6 M to the binding buffer. After centrifugation (20 000×g, 30 min, 4°C) the supernatant was passed through a 0.45-μM filter. The His-tagged ACUH was purified by Ni2+ affinity chromatography (Novagen) according to manufacturer's instructions using as elution buffer (6 M urea, 0.6 M imidazole, 500 mM NaCl, 20 mM Tris–HCl pH 7.9). Aliquots from different purification stages were separated in 10% SDS–PAGE and visualised with Coomassie staining.
2.5 Production and characterisation of the anti-ACUH serum
The purified His-tagged ACUH was used to immunise two New Zealand white rabbits. Characterisation of the anti-ACUH serum was performed by Western blotting. Proteins from E. coli over-expressing cells and the mitochondrial and peroxisomal (17 000×g) enriched fractions [19] of A. nidulans R21 and T1 strain were separated in a 10% SDS–PAGE and blotted onto a nitrocellulose membrane. Alkaline phosphatase-conjugated goat anti-rabbit IgG (Bio-Rad) were the secondary antibodies. The immune complexes were visualised using 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium.
2.6 Over-expression of ACUH in A. nidulans
Primers LKPNACUH (5′-CCTACATCCGGTACCCGCTTCAGC-3′) and RBAMACUH2 (5′-AATAAGGATCCGTCGGGGGCCT-3′), including a KpnI and a BamHI site respectively, were used in a PCR reaction to amplify a genomic DNA fragment from nucleotides −35 to +1496 of the acuH gene [1]. The PCR-amplified fragment including the complete acuH ORF plus 35 bp and 357 bp of its 5′ and 3′ flanking region was subcloned into pGEMTeasy. The KpnI–BamHI insert was cloned into pAL3 and the resultant plasmid, pALACUH, allowing expression of the acuH gene from its own start codon, was used to transform A. nidulans G191 using established procedures [20]. Eight pyrG+ transformants were further purified.
Genomic DNA from the recipient strain G191 and the eight transformants selected was isolated and digested with BglII, which does not cut within the vector pALACUH, or HindIII. Digests were separated in a 0.7% TAE-agarose gel, blotted onto a Hybond NX membrane (Amersham Pharmacia Biotech) and hybridised using the 1.5-kb KpnI–BamHI fragment containing the acuH gene as a probe. The T1 transformant that contains two copies of the alcAp–acuH fusion gene integrated at the acuH locus (not shown) was selected.
2.7 Electron immunocytochemistry
Hyphae were processed for immunocytochemical studies as described elsewhere [21]. Polyclonal antibodies against ACUH were used at 1/500 dilution in PBSG buffer (8 mM Na2HPO4, 1 mM NaH2PO4, 135 mM NaCl, 2 mM KCl, 20 mM glycine pH 9.0) containing 0.5% BSA. As the secondary antibodies, goat anti-rabbit IgG conjugated with 15-nm gold particles (Amersham Pharmacia Biotech) were used at 1/20 dilution in PBSG buffer. Sections were examined by microscopy using a Zeiss EM-10C transmission electron microscope operating at 60 kV.
3 Results
3.1 Bacterial over-expression and purification of ACUH
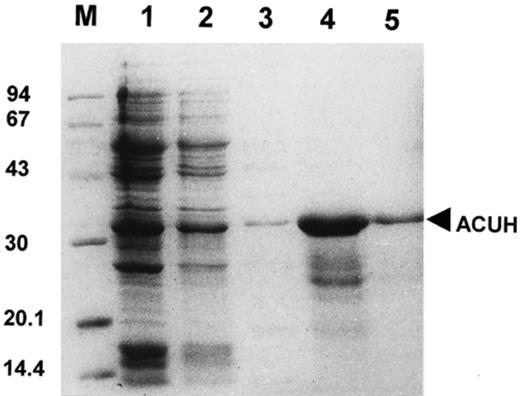
To over-express ACUH in E. coli the plasmid pD1ACUH which encodes the ACUH protein with a histidine tail at the N-terminus was constructed (see Section 2). E. coli BL21 (DE3) cells containing pD1ACUH were grown on LB medium. When the culture reached an OD600 of 0.7, cells were induced for 3 h by addition of IPTG. Results obtained in this experiment demonstrated the failure in over-expressing the His-tagged ACUH protein. Nevertheless, over-expression of the recombinant ACUH using the same growth conditions was achieved when E. coli BL21 (DE3) cells harbouring pD1ACUH were transformed with pGroESL encoding the GroEL and GroES heat-shock proteins [16] (Fig. 1, lane 1).
Purification of the over-expressed A. nidulans carnitine carrier from E. coli cells. Proteins were separated by SDS–PAGE and visualised with Coomassie staining. M, molecular mass markers (kDa). Lane 1, total protein from E. coli over-expressing cells. Lane 2, insoluble fraction of E. coli over-expressing cells. Lanes 3, 4 and 5, protein elution from the His-bind resin with 0.1, 1 and 0.6 M imidazole elution buffer, respectively. Purified ACUH (33 kDa) (lane 5).
Solubilisation of the His-tagged ACUH from the insoluble cell fraction was performed by adding 6 M urea. The recombinant protein was then purified by Ni2+ affinity chromatography under denaturing conditions (Fig. 1). Using the following elution buffer (6 M urea, 0.6 M imidazole, 500 mM NaCl, 20 mM Tris–HCl pH 7.9) a highly pure preparation of the His-tagged ACUH was obtained (Fig. 1, lane 5) as judged by the Coomassie staining of the sample.
3.2 Detection of ACUH by Western blotting
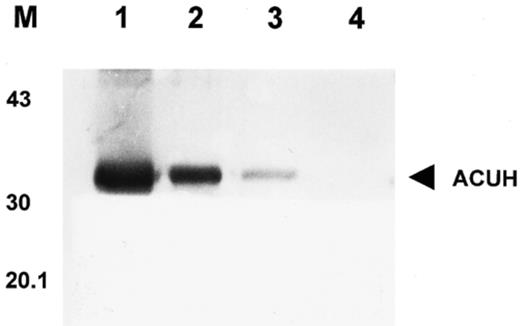
The purified ACUH was employed to immunise two rabbits where polyclonal antibodies against the A. nidulans carnitine carrier were raised. The specificity of the polyclonal antibodies was then analysed by Western blotting (Fig. 2).
Detection of ACUH by Western blotting. Lane 1, total protein (10 μg) from E. coli over-expressing cells. Lanes 2 and 3, mitochondrial and peroxisomal (17 000×g) enriched fraction (250 μg) from A. nidulans R21 (wild-type) (lane 3) and T1 over-expressing strain (lane 2) grown under inducing conditions. Lane 4, mitochondrial and peroxisomal enriched fraction (250 μg) of T1 over-expressing strain grown on glucose minimal medium. M, position of the molecular mass markers in kDa.
The anti-ACUH serum detected a single protein band of 33 kDa (the correct molecular mass) in the mitochondrial and peroxisomal enriched fraction of A. nidulans T1 over-expressing strain grown under inducing conditions (Fig. 2, lane 2). A much fainter band of similar molecular mass was also recognised by the anti-ACUH serum in the mitochondrial and peroxisomal enriched fraction of A. nidulans wild-type strain grown under inducing conditions (Fig. 2, lane 3). As expected, these antibodies failed to detect any protein band in the 17 000×g enriched fraction of T1 transformant incubated under repressing conditions (Fig. 2, lane 4). These results validated the utilisation of the anti-ACUH serum raised for localisation studies by electron immunocytochemistry.
3.3 Subcellular location of ACUH in A. nidulans
A. nidulans wild-type strain was incubated in 6 mM oleate minimal medium. The oleate medium induces expression of ACUH as well as a massive proliferation of peroxisomes [1,19]. Induced hyphae were utilised for localisation of the carnitine/acyl-carnitine carrier using the polyclonal antibodies raised and the immunogold technique.
The finding that only very few mitochondria (about 1 out of 50 observed) were labelled by the anti-ACUH serum (not shown), was to be expected because of the very low representation of mitochondrial carriers in cells. To overcome this problem an over-expression system for ACUH in A. nidulans was developed. We put the acuH gene under control of the strong promoter of the alcohol dehydrogenase gene (alcAp) [22] and induced the expression of this alcAp–acuH fusion gene by addition of threonine to the medium [23].
Plasmid pALACUH containing the alcAp–acuH fusion gene was used to transform the pyrG89 strain A. nidulans G191. The T1 transformant, which contains two copies of the alcAp–acuH fusion gene integrated at the acuH locus, was selected (see Materials and methods) and Western blotting analysis (Fig. 2) showing over-expression of ACUH in T1 cells validated the utilisation of this A. nidulans over-expressing strain for immunolocalisation studies.
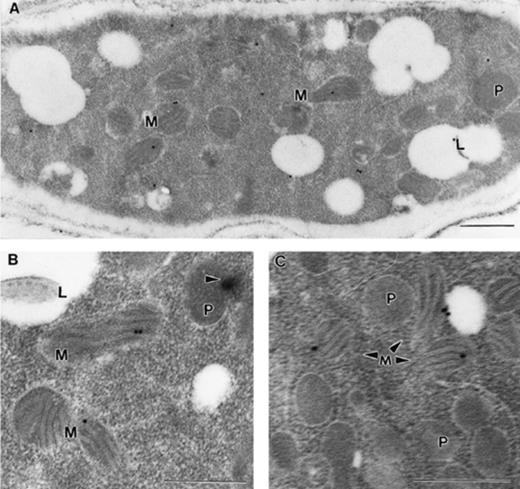
The T1 strain was incubated under inducing conditions (minimal medium with 0.1 M threonine and 6 mM oleate) allowing over-expression of ACUH and proliferation of peroxisomes. Ultrastructure of T1 induced cells subjected to the immunogold technique was well preserved, showing the presence of numerous clusters of peroxisomes, mitochondria and lipid droplets within the hyphae (Fig. 3). Although not all mitochondria of the T1 strain were labelled with the anti-ACUH serum (Fig. 3A), our results clearly demonstrate the localisation of the acuH gene product in the mitochondria. As can be seen (Fig. 3A–C), the cristae of several mitochondria were labelled with two or more gold particles, which clearly showed the localisation of the carnitine/acyl-carnitine translocase in the mitochondrial inner membrane.
Localisation in the mitochondrial inner membrane of the ACUH. A. nidulans T1 over-expressing strain grown in 0.1 M threonine+6 mM oleate minimal medium (inducing conditions) was processed for immunoelectron microscopy. A: Longitudinal section of a representative hyphae. Note that cristae of three mitochondria are labelled. The cytosolic labelling is probably non-specific. B: Detailed section demonstrating the localisation of ACUH in the mitochondrial inner membrane. Note the formation of a Woronin body (arrowhead) from the peroxisome. C: Detailed section showing the presence of numerous peroxisomes in T1 induced cells. The immunostaining is restricted to the mitochondrial inner membrane and not to the peroxisomes. Bars: 0.5 μm. M, mitochondrion; P, peroxisome; L lipid body.
Since we found no label in the peroxisomal membrane of the numerous peroxisomes visualised (Fig. 3A–C) in more than 1000 sections analysed, the hypothetical peroxisomal localisation of ACUH was ruled out. These immunocytochemical studies definitively demonstrate that in A. nidulans the carnitine/acyl-carnitine carrier encoded by the acuH gene is exclusively located in the mitochondrial inner membrane.
4 Discussion
In A. nidulans the catabolism of fatty acids begins with the peroxisomal fatty acid β-oxidation that produces acetyl-CoA. This acetyl-CoA must be transported from the peroxisomes to the mitochondrial matrix for final oxidation in the Krebs cycle [1]. Since the peroxisomal and mitochondrial inner membrane are impermeable to acyl-CoA esters including acetyl-CoA [8,9,24], we proposed in A. nidulans the existence of two functionally similar carnitine carriers located in both membranes for the transport of acetyl-CoA as acetyl-carnitine from the peroxisomes to the mitochondrial matrix [1].
In mammalian cells, the catabolism of fatty acids is more complex than in eukaryotic microorganisms and involves two different fatty acid β-oxidation systems, the peroxisomal and the mitochondrial fatty acid β-oxidation. Nevertheless, this metabolic pathway also requires the existence of both carnitine carriers for the transport of acyl-CoA esters of different length as acyl-carnitines across the peroxisomal and mitochondrial inner membrane [8].
The mitochondrial carnitine carrier, a protein belonging to the mitochondrial carrier family, has been identified in a few lower and higher eukaryotes [1,2,5,6,25;26;27]. However, no candidate gene encoding the peroxisomal carnitine carrier has yet been identified in any eukaryote. Here, we studied whether in A. nidulans the acuH gene encodes a carnitine carrier that is targeted to both organelles, mitochondria and peroxisomes, as recently suggested for the rat carnitine carrier [28]. To answer this question, specific antibodies against ACUH in combination with the most accurate technique for protein localisation, the immunogold technique, were employed.
To raise polyclonal antibodies against ACUH, we purified this protein from E. coli cells over-expressing a His-tagged version of ACUH. As described for other heterologous proteins [16], over-expression of ACUH in E. coli depends on over-expression of chaperonins GroEL and GroES encoded by the operon groE. This result suggests the participation of the groE operon products in the folding and stabilisation of the ACUH expressed in the system used in this work.
To determine the subcellular location of ACUH by electron immunocytochemistry, an A. nidulans over-expressing strain (T1 transformant) was derived in which the acuH gene was put under control of the strong promoter alcAp. Although the expression level of ACUH in this strain was sufficient for establishing its subcellular location, we expected to see higher representation of this carrier in a strain (T1 transformant) that contains two copies of the alcAp–acuH fusion gene. In A. nidulans, it was reported that a metabolite derived from the metabolism of acetate partially represses alcohol dehydrogenase (the alcA gene product) [23,29]. Since in our experiments, oleate had to be added to the threonine containing medium in order to induce the proliferation of peroxisomes as well as the expression of the alcAp–acuH fusion gene, it is likely that the same metabolite, also formed during the catabolism of oleate, could act as a repressor of ACUH in T1 cells.
Localisation studies have clearly demonstrated the exclusive targeting of the acuH gene product to the mitochondrial inner membrane. This result agrees with the mitochondrial location recently proposed for the Saccharomyces cerevisiae Yor100cp, the ACUH orthologue, by immunolocalisation of an NH epitope fused to its N-terminus [25]. Taken together, both results strongly suggests that, at least in lower eukaryotes, the gene encoding the carnitine/acyl-carnitine translocase, homologue of the cact human gene, encodes a protein that is exclusively targeted to the mitochondrial inner membrane.
In the model fungus A. nidulans there are still several acu (acetate and oleate utilisation) genes of unknown function and proposed to encode transporters specifically required during growth on fatty acids and acetate [1,7,18]. Cloning and characterisation of one of these acu genes might identify the gene encoding the peroxisomal carrier involved in the transport of acetyl-CoA molecules, as acetyl-carnitine, from the peroxisomes to the cytosol.
Acknowledgements
We thank Dr. Miguel Peñalva (Centro de Investigaciones Biológicas, CSIC, Madrid, Spain) for suggestions for the over-expression of ACUH in E. coli. This work was supported by the Consejería de Educación de la Comunidad de Madrid (Madrid, Spain) Project ref. 08.6/0012.1/1999 and 08.6/0039./20000. Also, the Spanish Ministerio de Ciencia y Tecnología (Project ref. PM99-0128) sponsored this study.
References