-
PDF
- Split View
-
Views
-
Cite
Cite
WANSHENG JIANG, HEOK HEE NG, JUNXING YANG, XIAOYONG CHEN, A taxonomic review of the catfish identified as Glyptothorax zanaensis (Teleostei: Siluriformes: Sisoridae), with the descriptions of two new species, Zoological Journal of the Linnean Society, Volume 165, Issue 2, June 2012, Pages 363–389, https://doi.org/10.1111/j.1096-3642.2011.00811.x
Close - Share Icon Share
Abstract
This study re-examined the taxonomic status of the sisorid catfishes usually identified as Glyptothorax zanaensis using a combination of morphometric and molecular data. Our results resurrect Glyptothorax longinema from the synonymy of G. zanaensis, and we describe two previously unnamed species as Glyptothorax granosus sp. nov. and Glyptothorax fucatus sp. nov. All four species are diagnosed and described in detail. Truss-based morphometrics combined with principal component analysis (PCA) detected three principal components (PCs) that can explain 86% of the total variation amongst species, which mainly reflect the characteristics of body depth, related depth, adhesive apparatus length, pectoral-fin length, caudal peduncle length, and barbel lengths. We also generated a phylogenetic hypothesis of these species using concatenated mitochondrial cytochrome b and D-loop gene sequences. Molecular dating analysis revealed a rapid speciation of Glyptothorax in the south-eastern corner of the Qinghai-Xizang Plateau from the middle Pliocene to early Pleistocene. A key to identify the Glyptothorax species from the Salween River drainage is also provided.
INTRODUCTION
Glyptothorax Blyth, 1860, is a speciose group of sisorid catfishes, with about 70 valid species (Ng & Hadiaty, 2009) found throughout much of the Asian continent. They occur from Asia Minor (in the Tigris and Euphrates river drainages) eastward to the Yangtze River drainage and southward to Sundaic South-East Asia (Ferraris, 2007; Ng & Kottelat, 2008). Typically found in fast flowing hill-streams or faster flowing reaches of larger rivers, Glyptothorax species are easily distinguished in having a thoracic adhesive apparatus comprising of an elliptical field of folded longitudinal pleats of skin. The genus is additionally diagnosed in having a detached distal portion of the premaxilla, and long and thin lateral arms of the vomer that extend under the entire length of the articular process of the lateral ethmoid (de Pinna, 1996).
Most studies on members of the genus have focused on the taxonomy of this group (particularly the descriptions of new taxa), although the phylogenetic relationships within the genus have been examined in recent studies (Jiang et al., 2011; Singh et al., 2011). Despite recent studies documenting the diversity within the genus, the taxonomy of Glyptothorax is still poorly resolved (Ng & Rachmatika, 2005). The situation can be mirrored for the species found in China, with only two revisionary studies (Li, 1984; Mo & Chu, 1986) being conducted. Although Chu & Mo (1999) is the most recent synopsis of Chinese Glyptothorax, their results follow essentially those of Mo & Chu (1986).
One of the more commonly encountered species in south-western China is Glyptothorax zanaensis. This species was originally described from Zana in Tibet, China, lying within the Nujiang (upper Salween) drainage (Wu, He & Chu, 1981). It has subsequently been reported from the Mekong River drainage as far south as Laos (Kottelat, 2001). In their revision of Chinese Glyptothorax, Mo & Chu (1986) synonymized Glyptothorax longinemaLi, 1984 and Glyptothorax rubermentusLi, 1984 with G. zanaensis without justification for their action.
Recent ichthyological surveys in the Irrawaddy, Salween, and Mekong River drainages in China have resulted in the collection of much additional material superficially resembling G. zanaensis, but whose exact identities could not be easily verified using existing literature. This became the impetus for us to re-examine the status of G. longinema and G. rubermentus, utilizing evidence from both morphological and molecular data. Our results revealed the existence of four species in the Salween and Mekong river drainages in China: G. zanaensis, G. longinema, and two previously unnamed species. This study redescribes G. zanaensis, revalidates and redescribes G. longinema, and describes the two unnamed species from the Nujiang (upper Salween) drainage as Glyptothorax granosus sp. nov. and Glyptothorax fucatus sp. nov.
MATERIAL AND METHODS
SAMPLING AND STORAGE
All specimens were preserved in 10% buffered formalin and then transferred to 75% ethanol for storage. Tissue samples were taken from the pelvic fin and preserved in 99.5% ethanol. Numbers within parentheses in listings of material examined refer to number of specimens examined, whereas those after a meristic count indicate number of specimens examined with that particular count. The institutional abbreviations for the repository of material used in this study follow those of Ferraris (2007), except for IHB in place of IHASW for the Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan. Although both abbreviations have been used in the literature, the former has been more frequently used recently. We therefore follow the prevailing trend in the use of IHB in place of IHASW.
MORPHOLOGICAL DATA ANALYSIS
Traditional measurements were made point to point with digital callipers and data recorded to nearest 0.1 mm. Counts and measurements were carried out on the left side of specimens whenever possible. Subunits of the head are presented as percentages of head length (% HL). Head length itself and measurements of body parts are given as percentages of standard length (% SL). The measurement methods largely follow Ng & Kottelat (1998), with some modifications following Jiang, Chen & Yang (2010).
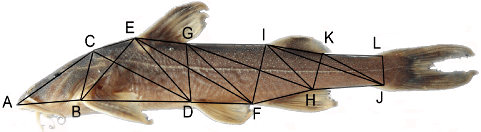
A truss-based morphometrics method was used in 76 individuals of the species treated in this study in order to further detect morphological differences. Twelve landmarks determining 25 distances were chosen and measured on the body, as illustrated in Figure 1. Landmarks refer to: (A) anterior tip of the snout on the upper jaw; (B) origin of pectoral fin; (C) tip of occipital bone; (D) origin of pelvic fin; (E) origin of dorsal fin; (F) origin of anal fin; (G) end of dorsal fin base; (H) end of anal fin base; (I) insertion of adipose fin; (J) insertion of lower caudal lobe; (K) end of adipose fin base; (L) insertion of upper caudal lobe. An additional 19 sets of morphometric data were also included in this analysis. A principal component analysis (PCA) of the above 44 morphological distances was implemented in SPSS 16.0 (SPSS for Windows, Chicago, IL, USA). All the variables were normalized with a log10 transformation before the PCA; a normal distribution test was then conducted to remove outlying data. The Bartlett's test of sphericity and Kaiser−Meyer−Olkin (KMO) test were carried out to determine whether the variables were suitable for exploratory factor analysis. Varimax rotation was utilized to simplify the factor loadings to better illustrate each component. This multivariate analysis allowed us to detect the combinations of characteristics that best illustrate morphological variation. Only undamaged fish were included in these measurements.
Lateral view of Glyptothorax granosus(KIZ 2000000555, paratype, 65.9 mm SL). Letters (A-L) show 12 landmarks which were used to determine 25 distances for the truss-based morphometric measurements.
MOLECULAR DATA ANALYSIS
Total DNA was purified from alcohol-preserved fin by the standard methods (Sambrook, Fritsch & Maniatis, 1989). Fragments of two mitochondrial genes, cytochrome b(cyt b) gene and control region (d-loop), were amplified using PCR using the following cycling conditions: an initial denaturing step at 95 °C for 4 min; 38 cycles of denaturing at 94 °C for 1 min, annealing at 52 °C for 1 min, and an extension at 72 °C for 1 min; with a final extension step of 72 °C for 10 min. The amplification primers of cyt b were L14724 (5'-GACTTGAAAAACCACCGTTG-3') and H15915 (5'-CTCCGATCTCCGGATTACAAGAC-3'), adapted from Xiao, Zhang & Liu (2001). The amplification primers of D-loop were DL1 (5'-ACCCCTGGCTCCCAAAGC-3') and DH2 (5'-ATCTTAGCATCTTCAGTG-3'), adapted from Liu, Tzeng & Teng (2002). PCR products were purified using the High Pure PCR Product Purification Kit (Bioteke) and sequenced in an automated DNA sequencer (ABI PRISM 3730) using the BigDye terminator v. 3.1, and following the manufacturer's instructions. All sequences have been deposited in GenBank (accession nos and details of specimen are listed inTable 1).
GenBank accession numbers of materials utilized in this study
| . | . | GenBank accession nos . | |
|---|---|---|---|
| Species name . | Locations . | Cyt b . | Dloop . |
| Bagarius yarrelli | NJ - Bizhai, Longlin County, Yunnan | HQ322525 | HQ322578 |
| Bagarius yarrelli | NJ - Bizhai, Longlin County, Yunnan | HQ322524 | HQ322577 |
| Glyptothorax obliquimaculatus | NJ - Mengding, Gengma County, Yunnan | HQ322553 | HQ322606 |
| Glyptothorax obliquimaculatus | NJ - Mengding, Gengma County, Yunnan | HQ322552 | HQ322605 |
| Glyptothorax burmanicus | NJ - Mengding, Gengma County, Yunnan | HQ322551 | HQ322604 |
| Glyptothorax burmanicus | NJ - Mengding, Gengma County, Yunnan | HQ322550 | HQ322603 |
| Glyptothorax burmanicus | NJ - Liuku to Fugong County, Yunnan | HQ322527 | HQ322580 |
| Glyptothorax laosensis | LCJ - Menglun, Xishuangbanna, Yunnan | HQ322523 | HQ322576 |
| Glyptothorax laosensis | LCJ - Manwan, Yun County, Yunnan | HQ322526 | HQ322579 |
| Glyptothorax trilineatus | NJ - Mengding, Gengma County, Yunnan | HQ322512 | HQ322565 |
| Glyptothorax trilineatus | NJ - Mengding, Gengma County, Yunnan | HQ322511 | HQ322564 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322520 | HQ322573 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322519 | HQ322572 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322518 | HQ322571 |
| Glyptothorax ngapang | NJ - Mucheng, Longlin County, Yunnan | HQ322530 | HQ322583 |
| Glyptothorax ngapang | NJ - Mengding, Gengma County, Yunnan | HQ322514 | HQ322567 |
| Glyptothorax ngapang | NJ - Mengding, Gengma County, Yunnan | HQ322548 | HQ322601 |
| Glyptothorax ngapang | NJ - Banlao, Cangyuan County, Yunnan | HQ322528 | HQ322581 |
| Glyptothorax fucatus sp. nov. | NJ - Banlao, Cangyuan County, Yunnan | HQ322515 | HQ322568 |
| Glyptothorax fucatus sp. nov. | NJ - Banlao, Cangyuan County, Yunnan | HQ322536 | HQ322589 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322516 | HQ322569 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322538 | HQ322591 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322537 | HQ322590 |
| Glyptothorax zanaensis | NJ - Dasuo, Gongshan County, Yunnan | HQ322542 | HQ322595 |
| Glyptothorax zanaensis | NJ - Dasuo, Gongshan County, Yunnan | HQ322541 | HQ322594 |
| Glyptothorax zanaensis | NJ - Hema, Gongshan County, Yunnan | HQ322540 | HQ322593 |
| Glyptothorax zanaensis | NJ - Shijia, Fugong to Gongshan County, Yunnan | HQ322507 | HQ322560 |
| Glyptothorax minimaculatus | LoCJ - Lianmengjie, Tengchong County, Yunnan | HQ322547 | HQ322600 |
| Glyptothorax minimaculatus | DYJ - Hehua, Tengchong County, Yunnan | HQ322535 | HQ322588 |
| Glyptothorax minimaculatus | DYJ - Hehua, Tengchong County, Yunnan | HQ322534 | HQ322587 |
| Glyptothorax granosus sp. nov. | NJ - Hema, Gongshan County, Yunnan | HQ322558 | HQ322611 |
| Glyptothorax granosus sp. nov. | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322506 | HQ322559 |
| Glyptothorax granosus sp. nov. | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322543 | HQ322596 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322522 | HQ322575 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322521 | HQ322574 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322508 | HQ322561 |
| Glyptothorax longinema | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322545 | HQ322598 |
| Glyptothorax longinema | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322544 | HQ322597 |
| Glyptothorax longinema | NJ - Daxingdi, Lushui County, Yunnan | HQ322510 | HQ322563 |
| Glyptothorax longinema | NJ - Daxingdi, Lushui County, Yunnan | HQ322546 | HQ322599 |
| Glyptothorax longinema | NJ - Dashaba, Lushui County, Yunnan | HQ322509 | HQ322562 |
| Glyptothorax longinema | NJ - Daojie, Baoshan City, Yunnan | HQ322557 | HQ322610 |
| Glyptothorax longinema | NJ - Bawan, Baoshan City, Yunnan | HQ322529 | HQ322582 |
| Glyptothorax longinema | NJ - Bizhai, Longlin County, Yunnan | HQ322533 | HQ322586 |
| Glyptothorax longinema | NJ - Bizhai, Longlin County, Yunnan | HQ322532 | HQ322585 |
| Glyptothorax longinema | NJ - Mengnuo, Longlin County, Yunnan | HQ322531 | HQ322584 |
| Glyptothorax longinema | NJ - Mengding, Gengma County, Yunnan | HQ322513 | HQ322566 |
| Glyptothorax longinema | NJ - Mengding, Gengma County, Yunnan | HQ322549 | HQ322602 |
| Glyptothorax longinema | LCJ - Wayao, Baoshan City, Yunnan | HQ322517 | HQ322570 |
| Glyptothorax longinema | LCJ - Wayao, Baoshan City, Yunnan | HQ322539 | HQ322592 |
| Glyptothorax longinema | LCJ - Jiuzhou, Yunlong County, Yunnan | HQ322556 | HQ322609 |
| Glyptothorax longinema | LCJ - Gongguoqiao, Yunlong County, Yunnan | HQ322555 | HQ322608 |
| Glyptothorax longinema | LCJ - Dachaoshan, Yun County, Yunnan | HQ322554 | HQ322607 |
| . | . | GenBank accession nos . | |
|---|---|---|---|
| Species name . | Locations . | Cyt b . | Dloop . |
| Bagarius yarrelli | NJ - Bizhai, Longlin County, Yunnan | HQ322525 | HQ322578 |
| Bagarius yarrelli | NJ - Bizhai, Longlin County, Yunnan | HQ322524 | HQ322577 |
| Glyptothorax obliquimaculatus | NJ - Mengding, Gengma County, Yunnan | HQ322553 | HQ322606 |
| Glyptothorax obliquimaculatus | NJ - Mengding, Gengma County, Yunnan | HQ322552 | HQ322605 |
| Glyptothorax burmanicus | NJ - Mengding, Gengma County, Yunnan | HQ322551 | HQ322604 |
| Glyptothorax burmanicus | NJ - Mengding, Gengma County, Yunnan | HQ322550 | HQ322603 |
| Glyptothorax burmanicus | NJ - Liuku to Fugong County, Yunnan | HQ322527 | HQ322580 |
| Glyptothorax laosensis | LCJ - Menglun, Xishuangbanna, Yunnan | HQ322523 | HQ322576 |
| Glyptothorax laosensis | LCJ - Manwan, Yun County, Yunnan | HQ322526 | HQ322579 |
| Glyptothorax trilineatus | NJ - Mengding, Gengma County, Yunnan | HQ322512 | HQ322565 |
| Glyptothorax trilineatus | NJ - Mengding, Gengma County, Yunnan | HQ322511 | HQ322564 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322520 | HQ322573 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322519 | HQ322572 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322518 | HQ322571 |
| Glyptothorax ngapang | NJ - Mucheng, Longlin County, Yunnan | HQ322530 | HQ322583 |
| Glyptothorax ngapang | NJ - Mengding, Gengma County, Yunnan | HQ322514 | HQ322567 |
| Glyptothorax ngapang | NJ - Mengding, Gengma County, Yunnan | HQ322548 | HQ322601 |
| Glyptothorax ngapang | NJ - Banlao, Cangyuan County, Yunnan | HQ322528 | HQ322581 |
| Glyptothorax fucatus sp. nov. | NJ - Banlao, Cangyuan County, Yunnan | HQ322515 | HQ322568 |
| Glyptothorax fucatus sp. nov. | NJ - Banlao, Cangyuan County, Yunnan | HQ322536 | HQ322589 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322516 | HQ322569 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322538 | HQ322591 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322537 | HQ322590 |
| Glyptothorax zanaensis | NJ - Dasuo, Gongshan County, Yunnan | HQ322542 | HQ322595 |
| Glyptothorax zanaensis | NJ - Dasuo, Gongshan County, Yunnan | HQ322541 | HQ322594 |
| Glyptothorax zanaensis | NJ - Hema, Gongshan County, Yunnan | HQ322540 | HQ322593 |
| Glyptothorax zanaensis | NJ - Shijia, Fugong to Gongshan County, Yunnan | HQ322507 | HQ322560 |
| Glyptothorax minimaculatus | LoCJ - Lianmengjie, Tengchong County, Yunnan | HQ322547 | HQ322600 |
| Glyptothorax minimaculatus | DYJ - Hehua, Tengchong County, Yunnan | HQ322535 | HQ322588 |
| Glyptothorax minimaculatus | DYJ - Hehua, Tengchong County, Yunnan | HQ322534 | HQ322587 |
| Glyptothorax granosus sp. nov. | NJ - Hema, Gongshan County, Yunnan | HQ322558 | HQ322611 |
| Glyptothorax granosus sp. nov. | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322506 | HQ322559 |
| Glyptothorax granosus sp. nov. | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322543 | HQ322596 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322522 | HQ322575 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322521 | HQ322574 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322508 | HQ322561 |
| Glyptothorax longinema | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322545 | HQ322598 |
| Glyptothorax longinema | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322544 | HQ322597 |
| Glyptothorax longinema | NJ - Daxingdi, Lushui County, Yunnan | HQ322510 | HQ322563 |
| Glyptothorax longinema | NJ - Daxingdi, Lushui County, Yunnan | HQ322546 | HQ322599 |
| Glyptothorax longinema | NJ - Dashaba, Lushui County, Yunnan | HQ322509 | HQ322562 |
| Glyptothorax longinema | NJ - Daojie, Baoshan City, Yunnan | HQ322557 | HQ322610 |
| Glyptothorax longinema | NJ - Bawan, Baoshan City, Yunnan | HQ322529 | HQ322582 |
| Glyptothorax longinema | NJ - Bizhai, Longlin County, Yunnan | HQ322533 | HQ322586 |
| Glyptothorax longinema | NJ - Bizhai, Longlin County, Yunnan | HQ322532 | HQ322585 |
| Glyptothorax longinema | NJ - Mengnuo, Longlin County, Yunnan | HQ322531 | HQ322584 |
| Glyptothorax longinema | NJ - Mengding, Gengma County, Yunnan | HQ322513 | HQ322566 |
| Glyptothorax longinema | NJ - Mengding, Gengma County, Yunnan | HQ322549 | HQ322602 |
| Glyptothorax longinema | LCJ - Wayao, Baoshan City, Yunnan | HQ322517 | HQ322570 |
| Glyptothorax longinema | LCJ - Wayao, Baoshan City, Yunnan | HQ322539 | HQ322592 |
| Glyptothorax longinema | LCJ - Jiuzhou, Yunlong County, Yunnan | HQ322556 | HQ322609 |
| Glyptothorax longinema | LCJ - Gongguoqiao, Yunlong County, Yunnan | HQ322555 | HQ322608 |
| Glyptothorax longinema | LCJ - Dachaoshan, Yun County, Yunnan | HQ322554 | HQ322607 |
DYJ, Dayingjiang drainage; LCJ, Lancangjiang drainage; LoCJ, Longchuanjiang drainage; NJ, Nujiang drainage.
GenBank accession numbers of materials utilized in this study
| . | . | GenBank accession nos . | |
|---|---|---|---|
| Species name . | Locations . | Cyt b . | Dloop . |
| Bagarius yarrelli | NJ - Bizhai, Longlin County, Yunnan | HQ322525 | HQ322578 |
| Bagarius yarrelli | NJ - Bizhai, Longlin County, Yunnan | HQ322524 | HQ322577 |
| Glyptothorax obliquimaculatus | NJ - Mengding, Gengma County, Yunnan | HQ322553 | HQ322606 |
| Glyptothorax obliquimaculatus | NJ - Mengding, Gengma County, Yunnan | HQ322552 | HQ322605 |
| Glyptothorax burmanicus | NJ - Mengding, Gengma County, Yunnan | HQ322551 | HQ322604 |
| Glyptothorax burmanicus | NJ - Mengding, Gengma County, Yunnan | HQ322550 | HQ322603 |
| Glyptothorax burmanicus | NJ - Liuku to Fugong County, Yunnan | HQ322527 | HQ322580 |
| Glyptothorax laosensis | LCJ - Menglun, Xishuangbanna, Yunnan | HQ322523 | HQ322576 |
| Glyptothorax laosensis | LCJ - Manwan, Yun County, Yunnan | HQ322526 | HQ322579 |
| Glyptothorax trilineatus | NJ - Mengding, Gengma County, Yunnan | HQ322512 | HQ322565 |
| Glyptothorax trilineatus | NJ - Mengding, Gengma County, Yunnan | HQ322511 | HQ322564 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322520 | HQ322573 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322519 | HQ322572 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322518 | HQ322571 |
| Glyptothorax ngapang | NJ - Mucheng, Longlin County, Yunnan | HQ322530 | HQ322583 |
| Glyptothorax ngapang | NJ - Mengding, Gengma County, Yunnan | HQ322514 | HQ322567 |
| Glyptothorax ngapang | NJ - Mengding, Gengma County, Yunnan | HQ322548 | HQ322601 |
| Glyptothorax ngapang | NJ - Banlao, Cangyuan County, Yunnan | HQ322528 | HQ322581 |
| Glyptothorax fucatus sp. nov. | NJ - Banlao, Cangyuan County, Yunnan | HQ322515 | HQ322568 |
| Glyptothorax fucatus sp. nov. | NJ - Banlao, Cangyuan County, Yunnan | HQ322536 | HQ322589 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322516 | HQ322569 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322538 | HQ322591 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322537 | HQ322590 |
| Glyptothorax zanaensis | NJ - Dasuo, Gongshan County, Yunnan | HQ322542 | HQ322595 |
| Glyptothorax zanaensis | NJ - Dasuo, Gongshan County, Yunnan | HQ322541 | HQ322594 |
| Glyptothorax zanaensis | NJ - Hema, Gongshan County, Yunnan | HQ322540 | HQ322593 |
| Glyptothorax zanaensis | NJ - Shijia, Fugong to Gongshan County, Yunnan | HQ322507 | HQ322560 |
| Glyptothorax minimaculatus | LoCJ - Lianmengjie, Tengchong County, Yunnan | HQ322547 | HQ322600 |
| Glyptothorax minimaculatus | DYJ - Hehua, Tengchong County, Yunnan | HQ322535 | HQ322588 |
| Glyptothorax minimaculatus | DYJ - Hehua, Tengchong County, Yunnan | HQ322534 | HQ322587 |
| Glyptothorax granosus sp. nov. | NJ - Hema, Gongshan County, Yunnan | HQ322558 | HQ322611 |
| Glyptothorax granosus sp. nov. | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322506 | HQ322559 |
| Glyptothorax granosus sp. nov. | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322543 | HQ322596 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322522 | HQ322575 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322521 | HQ322574 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322508 | HQ322561 |
| Glyptothorax longinema | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322545 | HQ322598 |
| Glyptothorax longinema | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322544 | HQ322597 |
| Glyptothorax longinema | NJ - Daxingdi, Lushui County, Yunnan | HQ322510 | HQ322563 |
| Glyptothorax longinema | NJ - Daxingdi, Lushui County, Yunnan | HQ322546 | HQ322599 |
| Glyptothorax longinema | NJ - Dashaba, Lushui County, Yunnan | HQ322509 | HQ322562 |
| Glyptothorax longinema | NJ - Daojie, Baoshan City, Yunnan | HQ322557 | HQ322610 |
| Glyptothorax longinema | NJ - Bawan, Baoshan City, Yunnan | HQ322529 | HQ322582 |
| Glyptothorax longinema | NJ - Bizhai, Longlin County, Yunnan | HQ322533 | HQ322586 |
| Glyptothorax longinema | NJ - Bizhai, Longlin County, Yunnan | HQ322532 | HQ322585 |
| Glyptothorax longinema | NJ - Mengnuo, Longlin County, Yunnan | HQ322531 | HQ322584 |
| Glyptothorax longinema | NJ - Mengding, Gengma County, Yunnan | HQ322513 | HQ322566 |
| Glyptothorax longinema | NJ - Mengding, Gengma County, Yunnan | HQ322549 | HQ322602 |
| Glyptothorax longinema | LCJ - Wayao, Baoshan City, Yunnan | HQ322517 | HQ322570 |
| Glyptothorax longinema | LCJ - Wayao, Baoshan City, Yunnan | HQ322539 | HQ322592 |
| Glyptothorax longinema | LCJ - Jiuzhou, Yunlong County, Yunnan | HQ322556 | HQ322609 |
| Glyptothorax longinema | LCJ - Gongguoqiao, Yunlong County, Yunnan | HQ322555 | HQ322608 |
| Glyptothorax longinema | LCJ - Dachaoshan, Yun County, Yunnan | HQ322554 | HQ322607 |
| . | . | GenBank accession nos . | |
|---|---|---|---|
| Species name . | Locations . | Cyt b . | Dloop . |
| Bagarius yarrelli | NJ - Bizhai, Longlin County, Yunnan | HQ322525 | HQ322578 |
| Bagarius yarrelli | NJ - Bizhai, Longlin County, Yunnan | HQ322524 | HQ322577 |
| Glyptothorax obliquimaculatus | NJ - Mengding, Gengma County, Yunnan | HQ322553 | HQ322606 |
| Glyptothorax obliquimaculatus | NJ - Mengding, Gengma County, Yunnan | HQ322552 | HQ322605 |
| Glyptothorax burmanicus | NJ - Mengding, Gengma County, Yunnan | HQ322551 | HQ322604 |
| Glyptothorax burmanicus | NJ - Mengding, Gengma County, Yunnan | HQ322550 | HQ322603 |
| Glyptothorax burmanicus | NJ - Liuku to Fugong County, Yunnan | HQ322527 | HQ322580 |
| Glyptothorax laosensis | LCJ - Menglun, Xishuangbanna, Yunnan | HQ322523 | HQ322576 |
| Glyptothorax laosensis | LCJ - Manwan, Yun County, Yunnan | HQ322526 | HQ322579 |
| Glyptothorax trilineatus | NJ - Mengding, Gengma County, Yunnan | HQ322512 | HQ322565 |
| Glyptothorax trilineatus | NJ - Mengding, Gengma County, Yunnan | HQ322511 | HQ322564 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322520 | HQ322573 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322519 | HQ322572 |
| Glyptothorax longjiangensis | LoCJ - Tenglongqiao, Longlin County, Yunnan | HQ322518 | HQ322571 |
| Glyptothorax ngapang | NJ - Mucheng, Longlin County, Yunnan | HQ322530 | HQ322583 |
| Glyptothorax ngapang | NJ - Mengding, Gengma County, Yunnan | HQ322514 | HQ322567 |
| Glyptothorax ngapang | NJ - Mengding, Gengma County, Yunnan | HQ322548 | HQ322601 |
| Glyptothorax ngapang | NJ - Banlao, Cangyuan County, Yunnan | HQ322528 | HQ322581 |
| Glyptothorax fucatus sp. nov. | NJ - Banlao, Cangyuan County, Yunnan | HQ322515 | HQ322568 |
| Glyptothorax fucatus sp. nov. | NJ - Banlao, Cangyuan County, Yunnan | HQ322536 | HQ322589 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322516 | HQ322569 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322538 | HQ322591 |
| Glyptothorax fucatus sp. nov. | NJ - Banhong, Cangyuan County, Yunnan | HQ322537 | HQ322590 |
| Glyptothorax zanaensis | NJ - Dasuo, Gongshan County, Yunnan | HQ322542 | HQ322595 |
| Glyptothorax zanaensis | NJ - Dasuo, Gongshan County, Yunnan | HQ322541 | HQ322594 |
| Glyptothorax zanaensis | NJ - Hema, Gongshan County, Yunnan | HQ322540 | HQ322593 |
| Glyptothorax zanaensis | NJ - Shijia, Fugong to Gongshan County, Yunnan | HQ322507 | HQ322560 |
| Glyptothorax minimaculatus | LoCJ - Lianmengjie, Tengchong County, Yunnan | HQ322547 | HQ322600 |
| Glyptothorax minimaculatus | DYJ - Hehua, Tengchong County, Yunnan | HQ322535 | HQ322588 |
| Glyptothorax minimaculatus | DYJ - Hehua, Tengchong County, Yunnan | HQ322534 | HQ322587 |
| Glyptothorax granosus sp. nov. | NJ - Hema, Gongshan County, Yunnan | HQ322558 | HQ322611 |
| Glyptothorax granosus sp. nov. | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322506 | HQ322559 |
| Glyptothorax granosus sp. nov. | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322543 | HQ322596 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322522 | HQ322575 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322521 | HQ322574 |
| Glyptothorax granosus sp. nov. | NJ - Daxingdi, Lushui County, Yunnan | HQ322508 | HQ322561 |
| Glyptothorax longinema | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322545 | HQ322598 |
| Glyptothorax longinema | NJ - Mabu River, Liuku to Fugong, Yunnan | HQ322544 | HQ322597 |
| Glyptothorax longinema | NJ - Daxingdi, Lushui County, Yunnan | HQ322510 | HQ322563 |
| Glyptothorax longinema | NJ - Daxingdi, Lushui County, Yunnan | HQ322546 | HQ322599 |
| Glyptothorax longinema | NJ - Dashaba, Lushui County, Yunnan | HQ322509 | HQ322562 |
| Glyptothorax longinema | NJ - Daojie, Baoshan City, Yunnan | HQ322557 | HQ322610 |
| Glyptothorax longinema | NJ - Bawan, Baoshan City, Yunnan | HQ322529 | HQ322582 |
| Glyptothorax longinema | NJ - Bizhai, Longlin County, Yunnan | HQ322533 | HQ322586 |
| Glyptothorax longinema | NJ - Bizhai, Longlin County, Yunnan | HQ322532 | HQ322585 |
| Glyptothorax longinema | NJ - Mengnuo, Longlin County, Yunnan | HQ322531 | HQ322584 |
| Glyptothorax longinema | NJ - Mengding, Gengma County, Yunnan | HQ322513 | HQ322566 |
| Glyptothorax longinema | NJ - Mengding, Gengma County, Yunnan | HQ322549 | HQ322602 |
| Glyptothorax longinema | LCJ - Wayao, Baoshan City, Yunnan | HQ322517 | HQ322570 |
| Glyptothorax longinema | LCJ - Wayao, Baoshan City, Yunnan | HQ322539 | HQ322592 |
| Glyptothorax longinema | LCJ - Jiuzhou, Yunlong County, Yunnan | HQ322556 | HQ322609 |
| Glyptothorax longinema | LCJ - Gongguoqiao, Yunlong County, Yunnan | HQ322555 | HQ322608 |
| Glyptothorax longinema | LCJ - Dachaoshan, Yun County, Yunnan | HQ322554 | HQ322607 |
DYJ, Dayingjiang drainage; LCJ, Lancangjiang drainage; LoCJ, Longchuanjiang drainage; NJ, Nujiang drainage.
Sequence chromatograms were viewed using SeqMan Pro (DNASTAR Inc., Madison, WI, USA) and verified by eye. Sequences were aligned with ClustalW (Thompson, Higgins & Gibson, 1994), with default gap penalties implemented as a tool in MEGA 4 (Tamura et al., 2007). Measures of nucleotide composition and a chi-square (χ2) test of homogeneity of base frequencies across taxa were obtained using PAUP* v. 4.0b10 (Swofford, 2003). The degree of heterogeneity between the two gene fragments was investigated using the partition homogeneity test (essentially the incongruence length difference test; Farris et al., 1995) in PAUP* with 1000 replicates and ten random sequence additions. A substitutional saturation test was implemented in DAMBE v. 5.1.2 (Xia & Xie, 2001), in which a plot of the transition and transversion rates versus the divergence offered a visual display.
Three methods were used to infer phylogenetic relationships: maximum parsimony (MP), maximum likelihood (ML), and Bayesian inference (BI). The parsimony analysis was performed with PAUP* and the trees were estimated using the heuristic search algorithm with tree-bisection-reconnection branch swapping and 100 random addition replicates, with all characters treated as equal weights and gaps as missing data. Maximum likelihood analyses were performed using the software PhyML v. 3.0 (Guindon & Gascuel, 2003). The most appropriate model for each data set was selected employing PAUP* and MODELTEST v. 3.7 (Posada & Crandall, 1998) under the Akaike information criterion, as recommended by Posada & Buckley (2004). Both the proportion of invariant sites and the gamma distribution parameter were fixed by the result of MODELTEST. Support for resolved clades was estimated using nonparametric bootstrapping (Felsenstein, 1985) with 1000 replications in both MP and ML analysis. MrBayes 3.1 (Huelsenbeck & Ronquist, 2001) was used to obtain the Bayesian tree. Posterior probabilities were based on two independent Markov chain Monte Carlo (MCMC) runs, each composed of four chains (three heated chains and one cold chain). The Markov chains were run for 2 × 106 generations, with sampling at every 100 generations yielding 20 000 trees. The first 5000 trees were discarded as nonconvergent.
Divergence times were estimated using BEAST 1.5.4 (Drummond & Rambaut, 2007). Both the best-fit model [general time reversible (GTR) + a proportion of invariable sites (I) + a gamma-shaped rate variation across sites (G)], as well as a relaxed clock model with a lognormal distribution and randomly generated starting trees with tree prior set to ‘Speciation-Yule Process’ were used in BEAUTi. Each run incorporated 4 × 106 generations with parameters logged every 1000th generation, and then the operators were adjusted following the suggestions of BEAST. Convergence was assessed in TRACER v. 1.4.1 (Rambaut & Drummond, 2007) and the effective sample sizes (ESSs) of parameters sampled from the final MCMC run were greater than 200. We used the only fossil record of sisorid fish, Bagarius yarelli from the Pliocene of the Siwalik Hills (Lydekker, 1886), as the sole calibration point; we followed Peng et al. (2006) in dating this point at 5.3 Mya.
Comparisons
Given that there are more than 70 species of Glyptothorax recognized as valid, with members of the genus having highly restricted distributions usually restricted to single drainages (Ferraris, 2007), we chose to compare the species treated in this study only with congeners known from the area of study, viz. the upper reaches of the Irrawaddy (Dayingjiang and Longchuanjiang), Mekong (Lancanjiang), and Salween (Nujiang) drainages in southern China. Besides the four species treated here, 11 species of Glyptothorax have been reported from this area: Glyptothorax burmanicus(Irrawaddy and Salween drainages), Glyptothorax deqinensis(Mekong drainage), Glyptothorax lampris(Mekong drainage), Glyptothorax laosensis(Mekong drainage), Glyptothorax longicauda(Irrawaddy drainage), Glyptothorax longjiangensis(Irrawaddy drainage), Glyptothorax macromaculatus(Mekong drainage), Glyptothorax minimaculatus(Irrawaddy drainage), Glyptothorax ngapang(Irrawaddy and Salween drainages), Glyptothorax obliquimaculatus(Salween drainage), and Glyptothorax trilineatus(Irrawaddy and Salween drainages). Glyptothorax burmanicus was often misidentified in earlier literature as Glyptothorax cavia, which is a species restricted to the Ganges−Brahmaputra system (Ng & Kottelat, 2008), whereas G. ngapang has often been misidentified in earlier literature as Glyptothorax dorsalis, a species known only from the lower Salween; it has also been misidentified in collections as G. minimaculatus(H-H. Ng, pers. observ.). Although G. lampris and G. laosensis have been recorded from the Lancangjiang (upper Mekong) drainage (Mo & Chu, 1986), there is evidence that these records might be based on material that is not conspecific with those from the middle and lower Mekong River drainage (Jiang et al., 2011). They are thus excluded from detailed comparisons in the diagnoses, but have been briefly compared in the Discussion.
RESULTS
Morphological variations by PCA
Both the Bartlett's test of sphericity and the KMO test showed that the variables were appropriate for factor analysis. Bartlett's test of sphericity rejected the null hypothesis (χ2= 6549, P= 0), which indicated that the correlation matrix was not an identity matrix. The value of the KMO test was 0.936, indicating that factor analysis would be able to extract a large amount of variance.
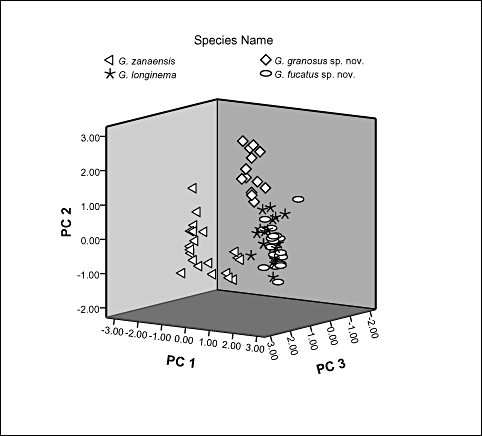
The initial eigenvalues of the first three principal components (PC) were greater than 1, which together explained 86% of the total variation amongst species. Covariance analysis showed that the three PCs were not correlated to each other (P= 0). A rotated component matrix was utilized to explain the correlation of variables and factors (Table 2). We considered the factor loadings of each variable higher than 0.80 to be highly correlated with the corresponding factor. PC1 accounted for 38% of the variation. It was loaded with the body depth, body depth at anus, interorbital distance, tip of occipital bone to origin of pelvic fin, origin of pelvic fin to end of dorsal fin base, origin of anal fin to insertion of adipose fin, end of anal fin base to end of adipose fin base, and insertion of lower to upper caudal lobe. In summary, it conveyed information on body depth and other measures relative to depth. PC2 accounted for 30% of the variation. It was loaded with adhesive apparatus length, pectoral-fin length, end of adipose fin base to insertion of upper caudal lobe, and end of anal fin base to insertion of lower caudal lobe. It conveyed information on fin, adhesive apparatus, and caudal peduncle lengths. PC3 accounted for 18% of the variation. It was loaded with length of nasal barbel, maxillary barbel, inner and outer mandibular barbels. It conveyed information on barbel lengths. The three-dimensional scatter plot of these three PCs clearly separated G. zanaensis and G. granosus sp. nov. from the grouping of G. longinema and G. fucatus sp. nov., but the latter two species could not be distinctly separated from each other (Fig. 2).
Factor loadings for the first three factors rotated component matrix from a principal component (PC) analysis of 44 variables of Glyptothorax fucatus, Glyptothorax granosus, Glyptothorax longinema, and Glyptothorax zanaensis
| Measurements . | PC 1 . | PC 2 . | PC 3 . |
|---|---|---|---|
| SL | 0.508 | 0.752 | 0.387 |
| BD | 0.861 | 0.374 | 0.121 |
| BDA | 0.926 | 0.318 | −0.013 |
| HL | 0.488 | 0.726 | 0.398 |
| HW | 0.736 | 0.506 | 0.361 |
| HD | 0.782 | 0.445 | 0.132 |
| MW | 0.464 | 0.165 | 0.782 |
| SnL | 0.509 | 0.732 | 0.292 |
| IOD | 0.859 | 0.112 | 0.318 |
| ED | 0.676 | 0.547 | −0.127 |
| NBL | 0.084 | 0.179 | 0.872 |
| MBL | 0.011 | 0.156 | 0.921 |
| IMBL | 0.074 | 0.111 | 0.914 |
| OMBL | 0.037 | 0.082 | 0.939 |
| AAL | 0.335 | 0.862 | −0.003 |
| AAW | 0.683 | 0.563 | 0.227 |
| PFL | 0.201 | 0.828 | 0.387 |
| VFL | 0.296 | 0.773 | 0.456 |
| CFL | 0.272 | 0.767 | 0.415 |
| A-B | 0.427 | 0.626 | 0.504 |
| A-C | 0.529 | 0.677 | 0.354 |
| B-C | 0.723 | 0.59 | 0.222 |
| B-D | 0.611 | 0.612 | 0.284 |
| B-E | 0.699 | 0.597 | 0.252 |
| C-D | 0.811 | 0.421 | 0.311 |
| C-E | 0.556 | 0.451 | 0.451 |
| D-E | 0.798 | 0.389 | 0.277 |
| D-G | 0.89 | 0.291 | 0.131 |
| D-F | 0.444 | 0.651 | 0.488 |
| E-G | 0.652 | 0.558 | 0.347 |
| E-F | 0.682 | 0.568 | 0.421 |
| F-G | 0.775 | 0.455 | 0.358 |
| F-H | 0.576 | 0.543 | 0.343 |
| F-I | 0.897 | 0.372 | 0.011 |
| G-H | 0.675 | 0.509 | 0.453 |
| G-I | 0.426 | 0.442 | 0.609 |
| H-I | 0.784 | 0.394 | 0.275 |
| H-K | 0.887 | 0.344 | 0.102 |
| I-K | 0.478 | 0.436 | 0.469 |
| I-J | 0.627 | 0.712 | 0.145 |
| J-K | 0.593 | 0.691 | −0.153 |
| K-L | 0.362 | 0.818 | −0.022 |
| H-J | 0.369 | 0.823 | 0.138 |
| J-L | 0.93 | 0.224 | 0.084 |
| Variances | 38.453 | 30.032 | 17.94 |
| Cumulative | 38.453 | 68.485 | 86.424 |
| Measurements . | PC 1 . | PC 2 . | PC 3 . |
|---|---|---|---|
| SL | 0.508 | 0.752 | 0.387 |
| BD | 0.861 | 0.374 | 0.121 |
| BDA | 0.926 | 0.318 | −0.013 |
| HL | 0.488 | 0.726 | 0.398 |
| HW | 0.736 | 0.506 | 0.361 |
| HD | 0.782 | 0.445 | 0.132 |
| MW | 0.464 | 0.165 | 0.782 |
| SnL | 0.509 | 0.732 | 0.292 |
| IOD | 0.859 | 0.112 | 0.318 |
| ED | 0.676 | 0.547 | −0.127 |
| NBL | 0.084 | 0.179 | 0.872 |
| MBL | 0.011 | 0.156 | 0.921 |
| IMBL | 0.074 | 0.111 | 0.914 |
| OMBL | 0.037 | 0.082 | 0.939 |
| AAL | 0.335 | 0.862 | −0.003 |
| AAW | 0.683 | 0.563 | 0.227 |
| PFL | 0.201 | 0.828 | 0.387 |
| VFL | 0.296 | 0.773 | 0.456 |
| CFL | 0.272 | 0.767 | 0.415 |
| A-B | 0.427 | 0.626 | 0.504 |
| A-C | 0.529 | 0.677 | 0.354 |
| B-C | 0.723 | 0.59 | 0.222 |
| B-D | 0.611 | 0.612 | 0.284 |
| B-E | 0.699 | 0.597 | 0.252 |
| C-D | 0.811 | 0.421 | 0.311 |
| C-E | 0.556 | 0.451 | 0.451 |
| D-E | 0.798 | 0.389 | 0.277 |
| D-G | 0.89 | 0.291 | 0.131 |
| D-F | 0.444 | 0.651 | 0.488 |
| E-G | 0.652 | 0.558 | 0.347 |
| E-F | 0.682 | 0.568 | 0.421 |
| F-G | 0.775 | 0.455 | 0.358 |
| F-H | 0.576 | 0.543 | 0.343 |
| F-I | 0.897 | 0.372 | 0.011 |
| G-H | 0.675 | 0.509 | 0.453 |
| G-I | 0.426 | 0.442 | 0.609 |
| H-I | 0.784 | 0.394 | 0.275 |
| H-K | 0.887 | 0.344 | 0.102 |
| I-K | 0.478 | 0.436 | 0.469 |
| I-J | 0.627 | 0.712 | 0.145 |
| J-K | 0.593 | 0.691 | −0.153 |
| K-L | 0.362 | 0.818 | −0.022 |
| H-J | 0.369 | 0.823 | 0.138 |
| J-L | 0.93 | 0.224 | 0.084 |
| Variances | 38.453 | 30.032 | 17.94 |
| Cumulative | 38.453 | 68.485 | 86.424 |
SL, standard length; BD, body depth; BDA, body depth at anus; HL, head length; HW, head width; HD, head depth; MW, mouth width; SnL, snout length; IOD, interorbital distance; ED, eye diameter; NBL, nasal barbel length; MBL, maxillary barbel length; IMBL, inner mandibular barbel length; OMBL, outer mandibular barbel length; AAL, adhesive apparatus length; AAW, adhesive apparatus width; PFL, pectoral-fin length; CFL, caudal-fin length; VFL, pelvic-fin length. Measurements from ‘Letter 1-Letter 2’ (e.g. A-B) refer to distances between landmarks (A to B) as illustrated in Figure 1. Bold font values signify factor loadings higher than 0.80.
Factor loadings for the first three factors rotated component matrix from a principal component (PC) analysis of 44 variables of Glyptothorax fucatus, Glyptothorax granosus, Glyptothorax longinema, and Glyptothorax zanaensis
| Measurements . | PC 1 . | PC 2 . | PC 3 . |
|---|---|---|---|
| SL | 0.508 | 0.752 | 0.387 |
| BD | 0.861 | 0.374 | 0.121 |
| BDA | 0.926 | 0.318 | −0.013 |
| HL | 0.488 | 0.726 | 0.398 |
| HW | 0.736 | 0.506 | 0.361 |
| HD | 0.782 | 0.445 | 0.132 |
| MW | 0.464 | 0.165 | 0.782 |
| SnL | 0.509 | 0.732 | 0.292 |
| IOD | 0.859 | 0.112 | 0.318 |
| ED | 0.676 | 0.547 | −0.127 |
| NBL | 0.084 | 0.179 | 0.872 |
| MBL | 0.011 | 0.156 | 0.921 |
| IMBL | 0.074 | 0.111 | 0.914 |
| OMBL | 0.037 | 0.082 | 0.939 |
| AAL | 0.335 | 0.862 | −0.003 |
| AAW | 0.683 | 0.563 | 0.227 |
| PFL | 0.201 | 0.828 | 0.387 |
| VFL | 0.296 | 0.773 | 0.456 |
| CFL | 0.272 | 0.767 | 0.415 |
| A-B | 0.427 | 0.626 | 0.504 |
| A-C | 0.529 | 0.677 | 0.354 |
| B-C | 0.723 | 0.59 | 0.222 |
| B-D | 0.611 | 0.612 | 0.284 |
| B-E | 0.699 | 0.597 | 0.252 |
| C-D | 0.811 | 0.421 | 0.311 |
| C-E | 0.556 | 0.451 | 0.451 |
| D-E | 0.798 | 0.389 | 0.277 |
| D-G | 0.89 | 0.291 | 0.131 |
| D-F | 0.444 | 0.651 | 0.488 |
| E-G | 0.652 | 0.558 | 0.347 |
| E-F | 0.682 | 0.568 | 0.421 |
| F-G | 0.775 | 0.455 | 0.358 |
| F-H | 0.576 | 0.543 | 0.343 |
| F-I | 0.897 | 0.372 | 0.011 |
| G-H | 0.675 | 0.509 | 0.453 |
| G-I | 0.426 | 0.442 | 0.609 |
| H-I | 0.784 | 0.394 | 0.275 |
| H-K | 0.887 | 0.344 | 0.102 |
| I-K | 0.478 | 0.436 | 0.469 |
| I-J | 0.627 | 0.712 | 0.145 |
| J-K | 0.593 | 0.691 | −0.153 |
| K-L | 0.362 | 0.818 | −0.022 |
| H-J | 0.369 | 0.823 | 0.138 |
| J-L | 0.93 | 0.224 | 0.084 |
| Variances | 38.453 | 30.032 | 17.94 |
| Cumulative | 38.453 | 68.485 | 86.424 |
| Measurements . | PC 1 . | PC 2 . | PC 3 . |
|---|---|---|---|
| SL | 0.508 | 0.752 | 0.387 |
| BD | 0.861 | 0.374 | 0.121 |
| BDA | 0.926 | 0.318 | −0.013 |
| HL | 0.488 | 0.726 | 0.398 |
| HW | 0.736 | 0.506 | 0.361 |
| HD | 0.782 | 0.445 | 0.132 |
| MW | 0.464 | 0.165 | 0.782 |
| SnL | 0.509 | 0.732 | 0.292 |
| IOD | 0.859 | 0.112 | 0.318 |
| ED | 0.676 | 0.547 | −0.127 |
| NBL | 0.084 | 0.179 | 0.872 |
| MBL | 0.011 | 0.156 | 0.921 |
| IMBL | 0.074 | 0.111 | 0.914 |
| OMBL | 0.037 | 0.082 | 0.939 |
| AAL | 0.335 | 0.862 | −0.003 |
| AAW | 0.683 | 0.563 | 0.227 |
| PFL | 0.201 | 0.828 | 0.387 |
| VFL | 0.296 | 0.773 | 0.456 |
| CFL | 0.272 | 0.767 | 0.415 |
| A-B | 0.427 | 0.626 | 0.504 |
| A-C | 0.529 | 0.677 | 0.354 |
| B-C | 0.723 | 0.59 | 0.222 |
| B-D | 0.611 | 0.612 | 0.284 |
| B-E | 0.699 | 0.597 | 0.252 |
| C-D | 0.811 | 0.421 | 0.311 |
| C-E | 0.556 | 0.451 | 0.451 |
| D-E | 0.798 | 0.389 | 0.277 |
| D-G | 0.89 | 0.291 | 0.131 |
| D-F | 0.444 | 0.651 | 0.488 |
| E-G | 0.652 | 0.558 | 0.347 |
| E-F | 0.682 | 0.568 | 0.421 |
| F-G | 0.775 | 0.455 | 0.358 |
| F-H | 0.576 | 0.543 | 0.343 |
| F-I | 0.897 | 0.372 | 0.011 |
| G-H | 0.675 | 0.509 | 0.453 |
| G-I | 0.426 | 0.442 | 0.609 |
| H-I | 0.784 | 0.394 | 0.275 |
| H-K | 0.887 | 0.344 | 0.102 |
| I-K | 0.478 | 0.436 | 0.469 |
| I-J | 0.627 | 0.712 | 0.145 |
| J-K | 0.593 | 0.691 | −0.153 |
| K-L | 0.362 | 0.818 | −0.022 |
| H-J | 0.369 | 0.823 | 0.138 |
| J-L | 0.93 | 0.224 | 0.084 |
| Variances | 38.453 | 30.032 | 17.94 |
| Cumulative | 38.453 | 68.485 | 86.424 |
SL, standard length; BD, body depth; BDA, body depth at anus; HL, head length; HW, head width; HD, head depth; MW, mouth width; SnL, snout length; IOD, interorbital distance; ED, eye diameter; NBL, nasal barbel length; MBL, maxillary barbel length; IMBL, inner mandibular barbel length; OMBL, outer mandibular barbel length; AAL, adhesive apparatus length; AAW, adhesive apparatus width; PFL, pectoral-fin length; CFL, caudal-fin length; VFL, pelvic-fin length. Measurements from ‘Letter 1-Letter 2’ (e.g. A-B) refer to distances between landmarks (A to B) as illustrated in Figure 1. Bold font values signify factor loadings higher than 0.80.
Three-dimensional scatterplot of principal components analysis showing morphological variations amongst the four species studied.
Molecular analysis
In order to recover phylogenetic relationships amongst material identified as G. zanaensis, we sequenced partial gene fragments of cyt b and D-loop using tissues from 62 individuals; sequences from seven other species of Glyptothorax as were added to the ingroup and those from Bagarius yarrelli used as the outgroup. The aligned cyt b sequence amongst the ingroup was 1138 bp in length and contained 353 variable sites, 260 of which were parsimony-informative. The aligned D-loop sequence amongst the ingroup was 1004 bp in length and contained 383 variable sites, 303 of which were parsimony-informative. The chi-square (χ2) test of homogeneity of base frequencies was calculated for each gene that did not demonstrate heterogeneity amongst the sequences. The results were as follows - cyt b: χ2= 15.30, d.f. = 156, P= 1.00; D-loop: χ2= 25.50, d.f. = 156, P= 1.00. Investigation of differences in incongruence length between cyt b and D-loop genes revealed that concatenating the partitions improved phylogenetic signal with a P-value of 0.09. Consequently, the concatenated data set was used for phylogenetic reconstruction. Evaluating the plots of pairwise comparisons of absolute numbers of transitions (TI) and transversions (TV) and corresponding TI/TV ratios versus F84 distances divergences indicated no saturation bias for any of the cyt b codon positions or D-loop (plots not shown).
Only the concatenated data set was used in all phylogenetic analyses. The MP analysis of the combined data set resulted in a total of 1000 equally parsimonious trees (gaps = missing, length = 1302, consistency index = 0.707, retention index = 0.896 with uninformative characters). The likelihood value of the ML tree was ln L = −9243.99, and the likelihood values of the consensus tree in the Bayesian approach were −9323.66 and −9330.47 for the cold chain of runs 1 and 2, respectively. Partitioned Bayesian analysis was used in the concatenated data set to reduce systematic error (Castoe & Parkinson, 2006). The GTR + I + G model was the most appropriate model for both cyt b and D-loop partitions, as indicated by MODELTEST v. 3.7.
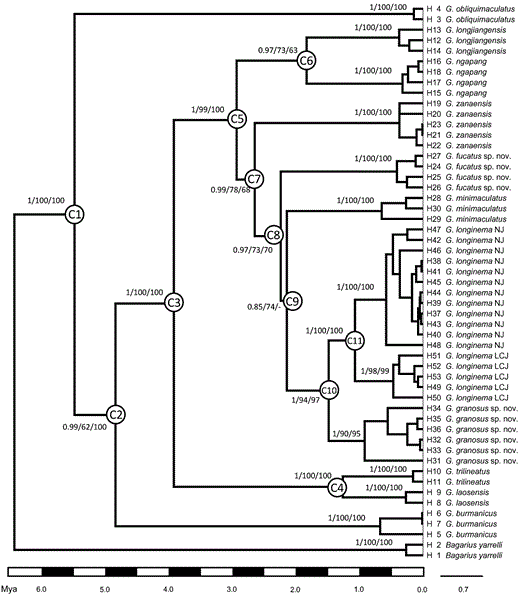
Topologies were very similar amongst MP, ML, and BI trees. As all clades utilized in BEAST followed the results of the BI analysis, only the Bayesian chronogram estimated in BEAST is presented here (Fig. 3). Phylogenetic trees showed that the four species studied here formed a monophyletic subclade (from node C7) with the inclusion of G. minimaculatus. Eleven nodes reflecting the divergence times of major groups were identified (Fig. 3). The upper and lower 95% confidence intervals and ESS scores are presented in Table 3. From node C7 the speciation of G. zanaensis and its closely related species occurred, which happened from the late Pliocene to early Pleistocene (about 2.34–1.21 Mya).
Bayesian inference chronogram showing the phylogeny and divergence time of Glyptothorax in the upper Irrawaddy (Dayingjiang and Longchuanjiang), Mekong (Lancangjiang), and Salween (Nujiang) drainages. Node C1 represents the calibration point and others denote nodes of interest. Support values of each node given as following: posterior probability/maximum likelihood bootstrap/maximum parsimony bootstrap. Abbreviations: LCJ, Lancangjiang drainage; NJ, Nujiang drainage.
Divergence times estimated from BEAST (nodes as indicated in Fig. 3)
| . | . | 95% confidence intervals . | . | |
|---|---|---|---|---|
| Node . | Mean TMRCA (Mya) . | Lower . | Upper . | ESS . |
| C1– | 5.16 | 4.15 | 6.15 | 8268.33 |
| C2 | 4.65 | 3.48 | 5.80 | 1279.58 |
| C3 | 3.43 | 2.33 | 4.53 | 260.75 |
| C4 | 1.56 | 0.40 | 2.80 | 239.59 |
| C5 | 2.68 | 1.68 | 3.71 | 251.10 |
| C6 | 1.76 | 0.68 | 2.91 | 274.80 |
| C7 | 2.34 | 1.43 | 3.30 | 249.34 |
| C8 | 2.04 | 1.19 | 2.93 | 236.84 |
| C9 | 1.93 | 1.13 | 2.82 | 235.44 |
| C10 | 1.62 | 0.93 | 2.43 | 221.84 |
| C11 | 1.21 | 0.61 | 1.87 | 216.68 |
| . | . | 95% confidence intervals . | . | |
|---|---|---|---|---|
| Node . | Mean TMRCA (Mya) . | Lower . | Upper . | ESS . |
| C1– | 5.16 | 4.15 | 6.15 | 8268.33 |
| C2 | 4.65 | 3.48 | 5.80 | 1279.58 |
| C3 | 3.43 | 2.33 | 4.53 | 260.75 |
| C4 | 1.56 | 0.40 | 2.80 | 239.59 |
| C5 | 2.68 | 1.68 | 3.71 | 251.10 |
| C6 | 1.76 | 0.68 | 2.91 | 274.80 |
| C7 | 2.34 | 1.43 | 3.30 | 249.34 |
| C8 | 2.04 | 1.19 | 2.93 | 236.84 |
| C9 | 1.93 | 1.13 | 2.82 | 235.44 |
| C10 | 1.62 | 0.93 | 2.43 | 221.84 |
| C11 | 1.21 | 0.61 | 1.87 | 216.68 |
Asterisk indicates estimate for node used as initial calibration point.
TMCRA, time since most recent common ancestor.
Confidence intervals are expressed in millions of years.
ESS is the effective sample size for the combined BEAST runs.
Divergence times estimated from BEAST (nodes as indicated in Fig. 3)
| . | . | 95% confidence intervals . | . | |
|---|---|---|---|---|
| Node . | Mean TMRCA (Mya) . | Lower . | Upper . | ESS . |
| C1– | 5.16 | 4.15 | 6.15 | 8268.33 |
| C2 | 4.65 | 3.48 | 5.80 | 1279.58 |
| C3 | 3.43 | 2.33 | 4.53 | 260.75 |
| C4 | 1.56 | 0.40 | 2.80 | 239.59 |
| C5 | 2.68 | 1.68 | 3.71 | 251.10 |
| C6 | 1.76 | 0.68 | 2.91 | 274.80 |
| C7 | 2.34 | 1.43 | 3.30 | 249.34 |
| C8 | 2.04 | 1.19 | 2.93 | 236.84 |
| C9 | 1.93 | 1.13 | 2.82 | 235.44 |
| C10 | 1.62 | 0.93 | 2.43 | 221.84 |
| C11 | 1.21 | 0.61 | 1.87 | 216.68 |
| . | . | 95% confidence intervals . | . | |
|---|---|---|---|---|
| Node . | Mean TMRCA (Mya) . | Lower . | Upper . | ESS . |
| C1– | 5.16 | 4.15 | 6.15 | 8268.33 |
| C2 | 4.65 | 3.48 | 5.80 | 1279.58 |
| C3 | 3.43 | 2.33 | 4.53 | 260.75 |
| C4 | 1.56 | 0.40 | 2.80 | 239.59 |
| C5 | 2.68 | 1.68 | 3.71 | 251.10 |
| C6 | 1.76 | 0.68 | 2.91 | 274.80 |
| C7 | 2.34 | 1.43 | 3.30 | 249.34 |
| C8 | 2.04 | 1.19 | 2.93 | 236.84 |
| C9 | 1.93 | 1.13 | 2.82 | 235.44 |
| C10 | 1.62 | 0.93 | 2.43 | 221.84 |
| C11 | 1.21 | 0.61 | 1.87 | 216.68 |
Asterisk indicates estimate for node used as initial calibration point.
TMCRA, time since most recent common ancestor.
Confidence intervals are expressed in millions of years.
ESS is the effective sample size for the combined BEAST runs.
TAXONOMY
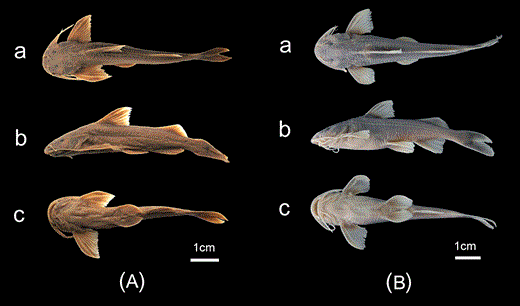
GLYPTOTHORAX ZANAENSISWU, HE & CHU, 1981(FIG. 4)
Glyptothorax zanaensis
Wu, He & Chu, 1981:74 (type locality: Zana, Tibet); Zhang et al., 1995: 130, fig. 57–1.
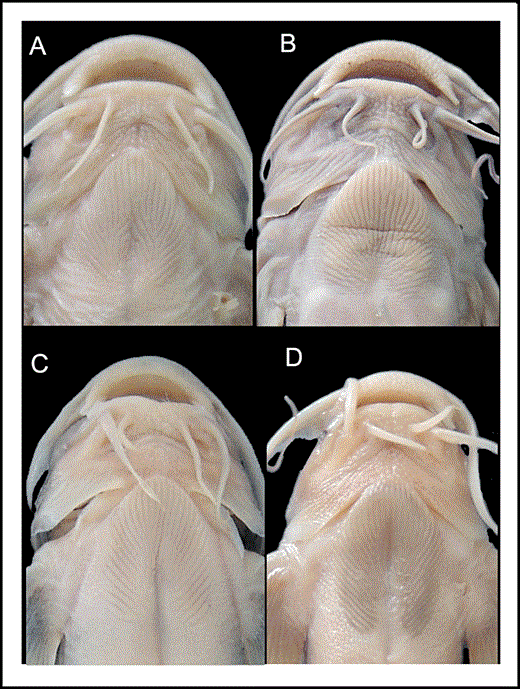
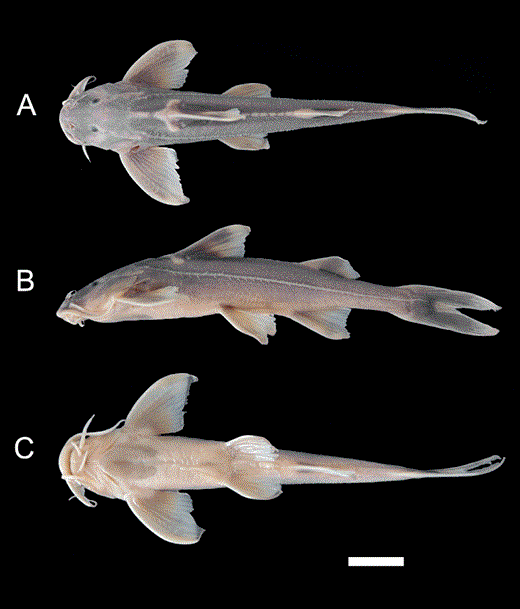
Glyptothorax zanaensis. A, IHB 6006653, syntype, 74.6 mm SL; China: Zana; B, KIZ 2000000534, 60.1 mm SL; China: Shangpa. a, dorsal view; b, lateral view; c, ventral view.
Glyptothorax zainaensis
Chu, Mo & Kuang, 1990:187 (in part); Chu & Mo, 1999: 146 (in part).
Material examined:
IHB 606164–606166 (three syntypes), 86.9–95.6 mm SL; IHB 606168 (one syntype), 92.3 mm SL; IHB 606170–606174 (five syntypes), 75.6–91.7 mm SL; IHB 606178 (one syntype), 75.3 mm SL; IHB 6006651–6006653 (three syntypes), 65.1–74.6 mm SL; IHB 606175 (1), 69 mm SL; China: Tibet, Changdu Prefecture, Zana. KIZ 2000000508 (1), 62.5 mm SL; KIZ 2000000517–2000000518 (2), 67.9–70.7 mm SL; KIZ 2000000520 (1), 71 mm SL; KIZ 2000000523 (1), 73.4 mm SL; KIZ 2000000529 (1), 65.6 mm SL; KIZ 2000000531 (1), 61.3 mm SL; China: Yunnan, Nujiang Prefecture, Gongshan County, Puladi Township. KIZ 2000000512 (1), 61.6 mm SL; KIZ 2000000536 (1), 54.7 mm SL; China: Yunnan, Nujiang Prefecture, Gongshan County, Cikai Township, 27°45'48''N, 98°42'39''E. KIZ 2000000534–2000000535 (2), 59.8–60.1 mm SL; China: Yunnan, Nujiang Prefecture, Fugong County, Shangpa Township, 26°55'10'N, 98°57'5'E. KIZ 2000000538 (1), 69.6 mm SL; China: Yunnan, Nujiang Prefecture, Fugong County, Zilijia Township, 26°42''N, 98°55''E. CAS 223336 (9), 61.0–75.7 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, west bank of Nujiang (Salween River), just north of Daliandi village, and 11 km south of Liuku on road to Shangjiang, 25°45'31.3''N, 98°51'48.3''E. CAS 223369 (15), 62.9–84.6 mm SL; China: Yunnan, Nujiang Prefecture, Gongshan County, Nujiang (Salween River) just below Yuange stream on east bank, c. 14 km south of bridge at Gongshan, 27°38'5.8''N, 98°43'51.2''E.
Diagnosis:
Glyptothorax zanaensis can be distinguished from other congeners in the upper Irrawaddy, Mekong, and Salween River drainages with a uniformly dark-coloured body in having a diverging pattern of striae running along the edges of the median depression in the thoracic adhesive apparatus (vs. such striae absent; Fig. 5). It further differs from G. burmanicus in having the depressed area in the thoracic adhesive apparatus not wholly enclosed by ridges (vs. ridges of the thoracic adhesive apparatus enclosing an ovoid depressed region in the centre) and a shorter head (23.3–26.7% SL vs. 27.6–30.8); from G. deqinensis in having more serrations on the posterior edge of the pectoral spine (nine to 13 vs. five to eight); from G. fucatus in having a uniformly coloured lateral surface of the body (vs. lateral surface of the body ventrally becoming paler immediately below lateral line), having a triangular anterior nuchal plate element with straight (vs. concave) anterolateral edges that lack (vs. with) extensive contact with the posterior nuchal plate element (Fig. 6), more slender body and caudal peduncle (depth at anus 11.4–15.8% SL vs. 15.5–21.1; depth of caudal peduncle 5.5–7.9% SL vs. 8.2–11.1), smaller eye (diameter 6.0–9.2% HL vs. 8.6–11.7), and longer nasal and maxillary barbels (length of nasal barbel 33.9–52.9% HL vs. 25.9–34.5; length of maxillary barbel: 103.8–161.0% HL vs. 77.2–109.0); and from G. granosus in having a triangular anterior nuchal plate element without (vs. with) saddle-shaped lateral expansions of the pterygiophores (Fig. 6), shorter post-adipose distance (12.0–16.4% SL vs. 15.7–21.6), caudal peduncle (14.8–18.9% SL vs. 18.2–23.7%), and thoracic adhesive apparatus (11.3–15.2% SL vs. 14.4–18.0), and longer barbels (length of nasal barbel 33.9–52.9% HL vs. 22.8–34.0; length of maxillary barbel 103.8–161.0% HL vs. 76.8–98.5; length of inner mandibular barbel 34.5–57.7% HL vs. 25.7–32.7; length of outer mandibular barbel 58.8–87.9% HL vs. 39.8–51.4). Glyptothorax zanaensis is further distinguished from G. longicauda in having a shorter caudal peduncle (14.8–18.9% SL vs. 17.9–24.9) and post-adipose distance (12.0–16.4% SL vs. 18.2–23.0), and fewer vertebrae (38–39 vs. 39–42), from G. longinema in having a more slender body and caudal peduncle (depth at anus 11.4–15.8% SL vs. 14.6–20.4; depth of caudal peduncle 5.5–7.9% SL vs. 7.1–10.6), from G. longjiangensis in having small, conical tubercles (vs. large plaques bearing unculiferous ridges) on the dorsal surface of the head, a shorter caudal peduncle (14.8–18.9% SL vs. 19.1–23.0), and longer nasal and maxillary barbels (length of nasal barbel 33.9–52.9% HL vs. 18.1–33.9; length of maxillary barbel: 103.8–161.0% HL vs. 65.8–102.8), and from G. macromaculatus in having distally expanded (vs. pointed) neural spines and a shorter head (23.3–26.7% SL vs. 27.4–34.4). It further differs from G. minimaculatus in lacking (vs. having) dark spots on the body and a smaller eye (diameter 6.0–9.2% HL vs. 10.2–12.4), from G. ngapang in having a longer adipose-fin base (14.1–19.6% SL vs. 10.7–14.0) and shorter caudal peduncle (14.8–18.9% SL vs. 18.7–23.6), from G. obliquimaculatus in lacking (vs. having) both the ridges of the thoracic adhesive apparatus extending onto the gular region and irregular dark blotches on the flanks and having a longer adipose-fin base (14.1–19.6% SL vs. 8.6–12.9) and more slender caudal peduncle (5.5–7.9% SL vs. 8.6–9.8), and from G. trilineatus in lacking (vs. having) a distinct pale midlateral line on the flank and having a shorter post-adipose distance (12.0–16.4% SL vs. 16.6–22.2), and longer nasal and maxillary barbels (length of nasal barbel 33.9–52.9% HL vs. 11.0–34.0; length of maxillary barbel 103.8–161.0% HL vs. 64.7–104.5).
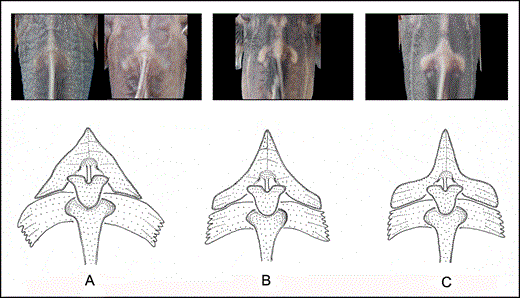
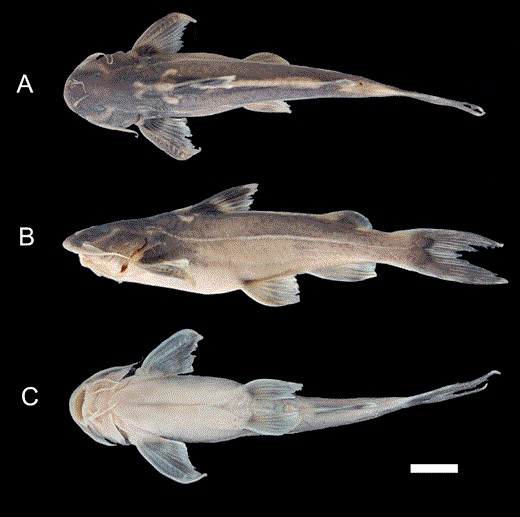
Thoracic adhesive apparatus of: A, Glyptothorax zanaensis, KIZ 2000000534, 60.1 mm SL; B, Glyptothorax longinema, KIZ 740197, 67 mm SL; C, Glyptothorax fucatus sp. nov., KIZ 20050410936, 72.9 mm SL, D, Glyptothorax granosus sp. nov., KIZ 2000000534, 61.8 mm SL.
Dorsal view of nuchal plate elements of: A, Glyptothorax zanaensis and Glyptothorax longinema; B, Glyptothorax fucatus sp. nov. ; and C, Glyptothorax granosus sp. nov.
Description:
Biometric data in Table 4.
Biometric data for Glyptothorax fucatus, Glyptothorax granosus, Glyptothorax longinema, and Glyptothorax zanaensis
| . | . | . | . | G. longinema(N= 29) . | . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | G. zanaensis(N= 26) . | . | Range . | . | G. fucatus sp. nov.(N= 27) . | G. granosus sp. nov.(N= 24) . | |||||||
| . | Holotype . | Range . | Mean ± SD . | Holotype . | Nujiang (N= 15) . | Lancangjiang (N= 14) . | Mean ± SD . | Holotype . | Range . | Mean ± SD . | Holotype . | Range . | Mean ± SD . |
| SL | 74.6 | 54.7–95.6 | 67.0 | 55.1–95.2 | 64.6–115.1 | 72.9 | 55.0–105.2 | 61.8 | 48.6–117.3 | ||||
| %SL | |||||||||||||
| PDL | 33.2 | 32.5–37.0 | 34.4 ± 1.21 | 37.0 | 32.4–38.7 | 33.6–38.7 | 35.6 ± 1.53 | 34.3 | 32.4–36.8 | 34.2 ± 1.09 | 36.1 | 31.6–37 | 34.7 ± 1.32 |
| PAL | 63.4 | 63.4–68.8 | 66.0 ± 1.44 | 64.9 | 63.7–68.1 | 64.1–68.1 | 66.1 ± 1.24 | 68.9 | 63.3–69.8 | 66.5 ± 1.83 | 63.3 | 59.7–66.3 | 63.3 ± 1.57 |
| PVL | 46.0 | 46.0–51.2 | 49.0 ± 1.41 | 49.0 | 46.4–49.9 | 47.2–51.6 | 48.8 ± 1.35 | 52.1 | 46.2–52.6 | 49.7 ± 1.64 | 46.1 | 44.8–49.7 | 47.5 ± 1.28 |
| PPL | 20.8 | 18.3–23.5 | 20.2 ± 1.41 | 20.6 | 18.2–23.6 | 16.0–22.8 | 19.3 ± 19.1 | 21.4 | 17.2–21.7 | 19.4 ± 1.12 | 17.5 | 17.3–21.2 | 19.1 ± 1.23 |
| LDFB | 11.5 | 10.6–13.7 | 12.2 ± 0.80 | 13.9 | 10.2–13.9 | 11.5–17.9 | 12.6 ± 1.67 | 11.9 | 10.9–14.3 | 12.1 ± 0.73 | 12.6 | 9.5–12.8 | 11.8 ± 0.82 |
| DSL | 16.2 | 12.3–16.8 | 15.2 ± 1.18 | 12.4 | 10.3–13.8 | 11.0–13.1 | 12.4 ± 0.90 | 15.8 | 11.7–16.4 | 14.4 ± 1.47 | 14.2 | 12.1–14.8 | 13.8 ± 0.81 |
| LAFB | 16.2 | 13.0–16.9 | 14.9 ± 0.98 | 14.5 | 12.3–15.9 | 12.9–17.2 | 14.6 ± 1.23 | 14.3 | 13.2–17.9 | 15.6 ± 1.52 | 14.2 | 11.8–15.2 | 13.7 ± 0.85 |
| VFL | 17.7 | 15.0–18.4 | 17.0 ± 0.90 | 17.0 | 14.4–17.1 | 14.9–17.9 | 16.3 ± 0.75 | 16.0 | 13.5–18.5 | 16.0 ± 1.15 | 16.2 | 14.0–18.8 | 16.6 ± 1.08 |
| PFL | 23.9 | 20.9–25.6 | 23.5 ± 1.23 | 22.5 | 20.0–26.1 | 20.2–25.1 | 22.9 ± 1.58 | 22.5 | 19.0–24.3 | 21.9 ± 1.59 | 25.4 | 22.8–26.5 | 24.5 ± 0.85 |
| PSL | 18.2 | 15.8–20.6 | 17.6 ± 1.32 | 17.5 | 13.0–18.8 | 13.9–19.6 | 16.8 ± 1.54 | 17.7 | 14.9–19.0 | 16.9 ± 1.23 | 18.0 | 16.5–19.7 | 18.1 ± 0.75 |
| CFL | 23.9 | 20.2–25.9 | 22.6 ± 1.37 | 23.9 | 19.3–25.6 | 20.2–23.7 | 22.3 ± 1.51 | 25.4 | 18.6–25.9 | 22.0 ± 1.82 | 24.3 | 20.5–25.9 | 23.5 ± 1.17 |
| LAdFB | 18.6 | 14.1–19.6 | 16.9 ± 1.46 | 16.0 | 13.1–17.8 | 13.5–17.4 | 15.3 ± 1.12 | 13.3 | 12.8–16.7 | 15.2 ± 1.15 | 14.9 | 12.8–15.9 | 14.4 ± 0.85 |
| DAD | 23.9 | 19.8–26.7 | 23.4 ± 2.08 | 23.4 | 20.3–26.2 | 21.8–26.2 | 23.0 ± 1.49 | 26.2 | 19.5–26.6 | 23.0 ± 1.98 | 20.9 | 16.7–23.6 | 20.7 ± 1.59 |
| PAD | 14.3 | 12.0–16.4 | 14.2 ± 1.21 | 14.6 | 14.2–18.0 | 14.3–18.2 | 15.9 ± 1.26 | 15.9 | 13.5–18.7 | 16.3 ± 1.33 | 17.5 | 15.7–21.6 | 19.2 ± 1.44 |
| LCP | 16.8 | 14.8–18.9 | 17.1 ± 1.16 | 17.6 | 17.3–20.7 | 16.8–21.9 | 18.7 ± 1.32 | 16.5 | 16.0–20.4 | 18.0 ± 1.33 | 20.1 | 18.2–23.7 | 21.4 ± 1.47 |
| DCP | 6.4 | 5.5–7.9 | 6.7 ± 0.50 | 8.4 | 7.1–10.6 | 7.4–10.1 | 8.9 ± 0.88 | 9.1 | 8.2–11.1 | 9.3 ± 0.88 | 7.0 | 5.7–7.6 | 6.7 ± 0.44 |
| BDA | 12.2 | 11.4–15.8 | 13.4 ± 1.32 | 17.3 | 14.6–20.4 | 15.9–19.9 | 17.2 ± 1.33 | 16.9 | 15.5–21.1 | 17.4 ± 1.28 | 13.9 | 12.9–16.5 | 14.1 ± 0.76 |
| HL | 24.9 | 23.3–26.7 | 24.8 ± 0.91 | 26.0 | 22.7–27.0 | 23.8–27.7 | 25.2 ± 1.17 | 24.1 | 21.7–25.6 | 24.1 ± 0.93 | 25.7 | 23.1–26.0 | 24.6 ± 0.80 |
| HW | 17.8 | 17.5–21.5 | 19.2 ± 1.09 | 20.9 | 19.4–22.8 | 19.3–22.9 | 20.7 ± 0.93 | 21.4 | 18.1–21.8 | 20.3 ± 0.94 | 19.1 | 16.6–19.5 | 18.1 ± 0.75 |
| HD | 12.7 | 12.3–16.7 | 14.6 ± 1.36 | 17.9 | 13.6–17.9 | 14.5–18.1 | 15.8 ± 1.32 | 16.0 | 14.4–17.4 | 16.0 ± 0.95 | 16.0 | 13.1–16.2 | 14.8 ± 0.94 |
| AAL | 14.2 | 11.3–15.2 | 13.7 ± 0.85 | 13.6 | 12.6–15.4 | 12.7–16.0 | 14.2 ± 1.04 | 14.7 | 11.9–15.7 | 13.9 ± 0.93 | 16.3 | 14.4–18.0 | 16.0 ± 0.89 |
| AAW | 9.5 | 7.9–11.4 | 9.5 ± 0.80 | 10.6 | 9.2–11.6 | 9.2–12.3 | 10.7 ± 0.78 | 10.0 | 8.9–11.4 | 10.2 ± 0.66 | 10.2 | 8.7–10.3 | 9.5 ± 0.51 |
| %HL | |||||||||||||
| SnL | 44.6 | 44.6–49.8 | 47.6 ± 1.41 | 49.4 | 45.8–52.4 | 44.1–54.0 | 49.7 ± 2.36 | 53.4 | 44.0–53.4 | 49.1 ± 2.43 | 49.7 | 44.9–52.7 | 49.4 ± 1.74 |
| IOD | 27.8 | 24.7–30.6 | 27.8 ± 2.02 | 31.0 | 25.0–31.2 | 23.3–29.5 | 27.8 ± 1.75 | 33.5 | 28.1–34.0 | 31.4 ± 1.42 | 25.2 | 20.4–28.8 | 23.9 ± 1.96 |
| ED | 7.0 | 6.0–9.2 | 7.8 ± 0.96 | 12.1 | 8.2–12.1 | 7.8–9.9 | 9.2 ± 0.87 | 10.2 | 8.6–11.7 | 9.8 ± 0.75 | 8.2 | 7.4–10.3 | 8.7 ± 0.79 |
| NBL | 40.3 | 33.9–52.9 | 40.6 ± 5.50 | 49.4 | 27.6–49.4 | 29.8–36.8 | 35.4 ± 4.80 | 28.4 | 25.9–34.5 | 30.0 ± 2.07 | 27.7 | 22.8–34.0 | 27.4 ± 2.73 |
| MBL | 132.8 | 103.8–161.0 | 124.2 ± 17.71 | 110.3 | 84.6–115.9 | 85.4–116.4 | 99.0 ± 10.11 | 89.8 | 77.2–109.0 | 95.7 ± 9.70 | 83.6 | 76.8–98.5 | 85.4 ± 5.70 |
| IMBL | 44.1 | 34.5–57.7 | 43.5 ± 5.52 | 36.2 | 29.1–42.8 | 30.9–41.9 | 34.1 ± 3.29 | 33.0 | 30.3–40.5 | 34.8 ± 2.63 | 27.0 | 25.7–32.7 | 29.1 ± 2.10 |
| OMBL | 75.3 | 58.8–87.9 | 69.6 ± 9.14 | 64.9 | 42.0–65.2 | 45.6–62.5 | 54.1 ± 6.10 | 54.5 | 46.6–65.6 | 55.5 ± 4.53 | 46.5 | 39.8–51.4 | 46.4 ± 3.14 |
| . | . | . | . | G. longinema(N= 29) . | . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | G. zanaensis(N= 26) . | . | Range . | . | G. fucatus sp. nov.(N= 27) . | G. granosus sp. nov.(N= 24) . | |||||||
| . | Holotype . | Range . | Mean ± SD . | Holotype . | Nujiang (N= 15) . | Lancangjiang (N= 14) . | Mean ± SD . | Holotype . | Range . | Mean ± SD . | Holotype . | Range . | Mean ± SD . |
| SL | 74.6 | 54.7–95.6 | 67.0 | 55.1–95.2 | 64.6–115.1 | 72.9 | 55.0–105.2 | 61.8 | 48.6–117.3 | ||||
| %SL | |||||||||||||
| PDL | 33.2 | 32.5–37.0 | 34.4 ± 1.21 | 37.0 | 32.4–38.7 | 33.6–38.7 | 35.6 ± 1.53 | 34.3 | 32.4–36.8 | 34.2 ± 1.09 | 36.1 | 31.6–37 | 34.7 ± 1.32 |
| PAL | 63.4 | 63.4–68.8 | 66.0 ± 1.44 | 64.9 | 63.7–68.1 | 64.1–68.1 | 66.1 ± 1.24 | 68.9 | 63.3–69.8 | 66.5 ± 1.83 | 63.3 | 59.7–66.3 | 63.3 ± 1.57 |
| PVL | 46.0 | 46.0–51.2 | 49.0 ± 1.41 | 49.0 | 46.4–49.9 | 47.2–51.6 | 48.8 ± 1.35 | 52.1 | 46.2–52.6 | 49.7 ± 1.64 | 46.1 | 44.8–49.7 | 47.5 ± 1.28 |
| PPL | 20.8 | 18.3–23.5 | 20.2 ± 1.41 | 20.6 | 18.2–23.6 | 16.0–22.8 | 19.3 ± 19.1 | 21.4 | 17.2–21.7 | 19.4 ± 1.12 | 17.5 | 17.3–21.2 | 19.1 ± 1.23 |
| LDFB | 11.5 | 10.6–13.7 | 12.2 ± 0.80 | 13.9 | 10.2–13.9 | 11.5–17.9 | 12.6 ± 1.67 | 11.9 | 10.9–14.3 | 12.1 ± 0.73 | 12.6 | 9.5–12.8 | 11.8 ± 0.82 |
| DSL | 16.2 | 12.3–16.8 | 15.2 ± 1.18 | 12.4 | 10.3–13.8 | 11.0–13.1 | 12.4 ± 0.90 | 15.8 | 11.7–16.4 | 14.4 ± 1.47 | 14.2 | 12.1–14.8 | 13.8 ± 0.81 |
| LAFB | 16.2 | 13.0–16.9 | 14.9 ± 0.98 | 14.5 | 12.3–15.9 | 12.9–17.2 | 14.6 ± 1.23 | 14.3 | 13.2–17.9 | 15.6 ± 1.52 | 14.2 | 11.8–15.2 | 13.7 ± 0.85 |
| VFL | 17.7 | 15.0–18.4 | 17.0 ± 0.90 | 17.0 | 14.4–17.1 | 14.9–17.9 | 16.3 ± 0.75 | 16.0 | 13.5–18.5 | 16.0 ± 1.15 | 16.2 | 14.0–18.8 | 16.6 ± 1.08 |
| PFL | 23.9 | 20.9–25.6 | 23.5 ± 1.23 | 22.5 | 20.0–26.1 | 20.2–25.1 | 22.9 ± 1.58 | 22.5 | 19.0–24.3 | 21.9 ± 1.59 | 25.4 | 22.8–26.5 | 24.5 ± 0.85 |
| PSL | 18.2 | 15.8–20.6 | 17.6 ± 1.32 | 17.5 | 13.0–18.8 | 13.9–19.6 | 16.8 ± 1.54 | 17.7 | 14.9–19.0 | 16.9 ± 1.23 | 18.0 | 16.5–19.7 | 18.1 ± 0.75 |
| CFL | 23.9 | 20.2–25.9 | 22.6 ± 1.37 | 23.9 | 19.3–25.6 | 20.2–23.7 | 22.3 ± 1.51 | 25.4 | 18.6–25.9 | 22.0 ± 1.82 | 24.3 | 20.5–25.9 | 23.5 ± 1.17 |
| LAdFB | 18.6 | 14.1–19.6 | 16.9 ± 1.46 | 16.0 | 13.1–17.8 | 13.5–17.4 | 15.3 ± 1.12 | 13.3 | 12.8–16.7 | 15.2 ± 1.15 | 14.9 | 12.8–15.9 | 14.4 ± 0.85 |
| DAD | 23.9 | 19.8–26.7 | 23.4 ± 2.08 | 23.4 | 20.3–26.2 | 21.8–26.2 | 23.0 ± 1.49 | 26.2 | 19.5–26.6 | 23.0 ± 1.98 | 20.9 | 16.7–23.6 | 20.7 ± 1.59 |
| PAD | 14.3 | 12.0–16.4 | 14.2 ± 1.21 | 14.6 | 14.2–18.0 | 14.3–18.2 | 15.9 ± 1.26 | 15.9 | 13.5–18.7 | 16.3 ± 1.33 | 17.5 | 15.7–21.6 | 19.2 ± 1.44 |
| LCP | 16.8 | 14.8–18.9 | 17.1 ± 1.16 | 17.6 | 17.3–20.7 | 16.8–21.9 | 18.7 ± 1.32 | 16.5 | 16.0–20.4 | 18.0 ± 1.33 | 20.1 | 18.2–23.7 | 21.4 ± 1.47 |
| DCP | 6.4 | 5.5–7.9 | 6.7 ± 0.50 | 8.4 | 7.1–10.6 | 7.4–10.1 | 8.9 ± 0.88 | 9.1 | 8.2–11.1 | 9.3 ± 0.88 | 7.0 | 5.7–7.6 | 6.7 ± 0.44 |
| BDA | 12.2 | 11.4–15.8 | 13.4 ± 1.32 | 17.3 | 14.6–20.4 | 15.9–19.9 | 17.2 ± 1.33 | 16.9 | 15.5–21.1 | 17.4 ± 1.28 | 13.9 | 12.9–16.5 | 14.1 ± 0.76 |
| HL | 24.9 | 23.3–26.7 | 24.8 ± 0.91 | 26.0 | 22.7–27.0 | 23.8–27.7 | 25.2 ± 1.17 | 24.1 | 21.7–25.6 | 24.1 ± 0.93 | 25.7 | 23.1–26.0 | 24.6 ± 0.80 |
| HW | 17.8 | 17.5–21.5 | 19.2 ± 1.09 | 20.9 | 19.4–22.8 | 19.3–22.9 | 20.7 ± 0.93 | 21.4 | 18.1–21.8 | 20.3 ± 0.94 | 19.1 | 16.6–19.5 | 18.1 ± 0.75 |
| HD | 12.7 | 12.3–16.7 | 14.6 ± 1.36 | 17.9 | 13.6–17.9 | 14.5–18.1 | 15.8 ± 1.32 | 16.0 | 14.4–17.4 | 16.0 ± 0.95 | 16.0 | 13.1–16.2 | 14.8 ± 0.94 |
| AAL | 14.2 | 11.3–15.2 | 13.7 ± 0.85 | 13.6 | 12.6–15.4 | 12.7–16.0 | 14.2 ± 1.04 | 14.7 | 11.9–15.7 | 13.9 ± 0.93 | 16.3 | 14.4–18.0 | 16.0 ± 0.89 |
| AAW | 9.5 | 7.9–11.4 | 9.5 ± 0.80 | 10.6 | 9.2–11.6 | 9.2–12.3 | 10.7 ± 0.78 | 10.0 | 8.9–11.4 | 10.2 ± 0.66 | 10.2 | 8.7–10.3 | 9.5 ± 0.51 |
| %HL | |||||||||||||
| SnL | 44.6 | 44.6–49.8 | 47.6 ± 1.41 | 49.4 | 45.8–52.4 | 44.1–54.0 | 49.7 ± 2.36 | 53.4 | 44.0–53.4 | 49.1 ± 2.43 | 49.7 | 44.9–52.7 | 49.4 ± 1.74 |
| IOD | 27.8 | 24.7–30.6 | 27.8 ± 2.02 | 31.0 | 25.0–31.2 | 23.3–29.5 | 27.8 ± 1.75 | 33.5 | 28.1–34.0 | 31.4 ± 1.42 | 25.2 | 20.4–28.8 | 23.9 ± 1.96 |
| ED | 7.0 | 6.0–9.2 | 7.8 ± 0.96 | 12.1 | 8.2–12.1 | 7.8–9.9 | 9.2 ± 0.87 | 10.2 | 8.6–11.7 | 9.8 ± 0.75 | 8.2 | 7.4–10.3 | 8.7 ± 0.79 |
| NBL | 40.3 | 33.9–52.9 | 40.6 ± 5.50 | 49.4 | 27.6–49.4 | 29.8–36.8 | 35.4 ± 4.80 | 28.4 | 25.9–34.5 | 30.0 ± 2.07 | 27.7 | 22.8–34.0 | 27.4 ± 2.73 |
| MBL | 132.8 | 103.8–161.0 | 124.2 ± 17.71 | 110.3 | 84.6–115.9 | 85.4–116.4 | 99.0 ± 10.11 | 89.8 | 77.2–109.0 | 95.7 ± 9.70 | 83.6 | 76.8–98.5 | 85.4 ± 5.70 |
| IMBL | 44.1 | 34.5–57.7 | 43.5 ± 5.52 | 36.2 | 29.1–42.8 | 30.9–41.9 | 34.1 ± 3.29 | 33.0 | 30.3–40.5 | 34.8 ± 2.63 | 27.0 | 25.7–32.7 | 29.1 ± 2.10 |
| OMBL | 75.3 | 58.8–87.9 | 69.6 ± 9.14 | 64.9 | 42.0–65.2 | 45.6–62.5 | 54.1 ± 6.10 | 54.5 | 46.6–65.6 | 55.5 ± 4.53 | 46.5 | 39.8–51.4 | 46.4 ± 3.14 |
SL, standard length; PDL, predorsal length; PAL, preanal length; PVL, prepelvic length; PPL, prepectoral length; LDFB, length of dorsal-fin base; DSL, dorsal-spine length; LAFB, length of anal-fin base; VFL, pelvic-fin length; PFL, pectoral-fin length; PSL, pectoral-spine length; CFL, caudal-fin length; LAdFB, length of adipose-fin base; DAD, dorsal to adipose distance; PAD, post-adipose distance; LCP, length of caudal peduncle; DCP, depth of caudal peduncle; BDA, body depth at anus; HL, head length; HW, head width; HD, head depth; AAL, adhesive apparatus length; AAW, adhesive apparatus width; SnL, snout length; IOD, interorbital distance; ED, eye diameter; NBL, nasal barbel length; MBL, maxillary barbel length; IMBL, inner mandibular barbel length; OMBL, outer mandibular barbel length.
Biometric data for Glyptothorax fucatus, Glyptothorax granosus, Glyptothorax longinema, and Glyptothorax zanaensis
| . | . | . | . | G. longinema(N= 29) . | . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | G. zanaensis(N= 26) . | . | Range . | . | G. fucatus sp. nov.(N= 27) . | G. granosus sp. nov.(N= 24) . | |||||||
| . | Holotype . | Range . | Mean ± SD . | Holotype . | Nujiang (N= 15) . | Lancangjiang (N= 14) . | Mean ± SD . | Holotype . | Range . | Mean ± SD . | Holotype . | Range . | Mean ± SD . |
| SL | 74.6 | 54.7–95.6 | 67.0 | 55.1–95.2 | 64.6–115.1 | 72.9 | 55.0–105.2 | 61.8 | 48.6–117.3 | ||||
| %SL | |||||||||||||
| PDL | 33.2 | 32.5–37.0 | 34.4 ± 1.21 | 37.0 | 32.4–38.7 | 33.6–38.7 | 35.6 ± 1.53 | 34.3 | 32.4–36.8 | 34.2 ± 1.09 | 36.1 | 31.6–37 | 34.7 ± 1.32 |
| PAL | 63.4 | 63.4–68.8 | 66.0 ± 1.44 | 64.9 | 63.7–68.1 | 64.1–68.1 | 66.1 ± 1.24 | 68.9 | 63.3–69.8 | 66.5 ± 1.83 | 63.3 | 59.7–66.3 | 63.3 ± 1.57 |
| PVL | 46.0 | 46.0–51.2 | 49.0 ± 1.41 | 49.0 | 46.4–49.9 | 47.2–51.6 | 48.8 ± 1.35 | 52.1 | 46.2–52.6 | 49.7 ± 1.64 | 46.1 | 44.8–49.7 | 47.5 ± 1.28 |
| PPL | 20.8 | 18.3–23.5 | 20.2 ± 1.41 | 20.6 | 18.2–23.6 | 16.0–22.8 | 19.3 ± 19.1 | 21.4 | 17.2–21.7 | 19.4 ± 1.12 | 17.5 | 17.3–21.2 | 19.1 ± 1.23 |
| LDFB | 11.5 | 10.6–13.7 | 12.2 ± 0.80 | 13.9 | 10.2–13.9 | 11.5–17.9 | 12.6 ± 1.67 | 11.9 | 10.9–14.3 | 12.1 ± 0.73 | 12.6 | 9.5–12.8 | 11.8 ± 0.82 |
| DSL | 16.2 | 12.3–16.8 | 15.2 ± 1.18 | 12.4 | 10.3–13.8 | 11.0–13.1 | 12.4 ± 0.90 | 15.8 | 11.7–16.4 | 14.4 ± 1.47 | 14.2 | 12.1–14.8 | 13.8 ± 0.81 |
| LAFB | 16.2 | 13.0–16.9 | 14.9 ± 0.98 | 14.5 | 12.3–15.9 | 12.9–17.2 | 14.6 ± 1.23 | 14.3 | 13.2–17.9 | 15.6 ± 1.52 | 14.2 | 11.8–15.2 | 13.7 ± 0.85 |
| VFL | 17.7 | 15.0–18.4 | 17.0 ± 0.90 | 17.0 | 14.4–17.1 | 14.9–17.9 | 16.3 ± 0.75 | 16.0 | 13.5–18.5 | 16.0 ± 1.15 | 16.2 | 14.0–18.8 | 16.6 ± 1.08 |
| PFL | 23.9 | 20.9–25.6 | 23.5 ± 1.23 | 22.5 | 20.0–26.1 | 20.2–25.1 | 22.9 ± 1.58 | 22.5 | 19.0–24.3 | 21.9 ± 1.59 | 25.4 | 22.8–26.5 | 24.5 ± 0.85 |
| PSL | 18.2 | 15.8–20.6 | 17.6 ± 1.32 | 17.5 | 13.0–18.8 | 13.9–19.6 | 16.8 ± 1.54 | 17.7 | 14.9–19.0 | 16.9 ± 1.23 | 18.0 | 16.5–19.7 | 18.1 ± 0.75 |
| CFL | 23.9 | 20.2–25.9 | 22.6 ± 1.37 | 23.9 | 19.3–25.6 | 20.2–23.7 | 22.3 ± 1.51 | 25.4 | 18.6–25.9 | 22.0 ± 1.82 | 24.3 | 20.5–25.9 | 23.5 ± 1.17 |
| LAdFB | 18.6 | 14.1–19.6 | 16.9 ± 1.46 | 16.0 | 13.1–17.8 | 13.5–17.4 | 15.3 ± 1.12 | 13.3 | 12.8–16.7 | 15.2 ± 1.15 | 14.9 | 12.8–15.9 | 14.4 ± 0.85 |
| DAD | 23.9 | 19.8–26.7 | 23.4 ± 2.08 | 23.4 | 20.3–26.2 | 21.8–26.2 | 23.0 ± 1.49 | 26.2 | 19.5–26.6 | 23.0 ± 1.98 | 20.9 | 16.7–23.6 | 20.7 ± 1.59 |
| PAD | 14.3 | 12.0–16.4 | 14.2 ± 1.21 | 14.6 | 14.2–18.0 | 14.3–18.2 | 15.9 ± 1.26 | 15.9 | 13.5–18.7 | 16.3 ± 1.33 | 17.5 | 15.7–21.6 | 19.2 ± 1.44 |
| LCP | 16.8 | 14.8–18.9 | 17.1 ± 1.16 | 17.6 | 17.3–20.7 | 16.8–21.9 | 18.7 ± 1.32 | 16.5 | 16.0–20.4 | 18.0 ± 1.33 | 20.1 | 18.2–23.7 | 21.4 ± 1.47 |
| DCP | 6.4 | 5.5–7.9 | 6.7 ± 0.50 | 8.4 | 7.1–10.6 | 7.4–10.1 | 8.9 ± 0.88 | 9.1 | 8.2–11.1 | 9.3 ± 0.88 | 7.0 | 5.7–7.6 | 6.7 ± 0.44 |
| BDA | 12.2 | 11.4–15.8 | 13.4 ± 1.32 | 17.3 | 14.6–20.4 | 15.9–19.9 | 17.2 ± 1.33 | 16.9 | 15.5–21.1 | 17.4 ± 1.28 | 13.9 | 12.9–16.5 | 14.1 ± 0.76 |
| HL | 24.9 | 23.3–26.7 | 24.8 ± 0.91 | 26.0 | 22.7–27.0 | 23.8–27.7 | 25.2 ± 1.17 | 24.1 | 21.7–25.6 | 24.1 ± 0.93 | 25.7 | 23.1–26.0 | 24.6 ± 0.80 |
| HW | 17.8 | 17.5–21.5 | 19.2 ± 1.09 | 20.9 | 19.4–22.8 | 19.3–22.9 | 20.7 ± 0.93 | 21.4 | 18.1–21.8 | 20.3 ± 0.94 | 19.1 | 16.6–19.5 | 18.1 ± 0.75 |
| HD | 12.7 | 12.3–16.7 | 14.6 ± 1.36 | 17.9 | 13.6–17.9 | 14.5–18.1 | 15.8 ± 1.32 | 16.0 | 14.4–17.4 | 16.0 ± 0.95 | 16.0 | 13.1–16.2 | 14.8 ± 0.94 |
| AAL | 14.2 | 11.3–15.2 | 13.7 ± 0.85 | 13.6 | 12.6–15.4 | 12.7–16.0 | 14.2 ± 1.04 | 14.7 | 11.9–15.7 | 13.9 ± 0.93 | 16.3 | 14.4–18.0 | 16.0 ± 0.89 |
| AAW | 9.5 | 7.9–11.4 | 9.5 ± 0.80 | 10.6 | 9.2–11.6 | 9.2–12.3 | 10.7 ± 0.78 | 10.0 | 8.9–11.4 | 10.2 ± 0.66 | 10.2 | 8.7–10.3 | 9.5 ± 0.51 |
| %HL | |||||||||||||
| SnL | 44.6 | 44.6–49.8 | 47.6 ± 1.41 | 49.4 | 45.8–52.4 | 44.1–54.0 | 49.7 ± 2.36 | 53.4 | 44.0–53.4 | 49.1 ± 2.43 | 49.7 | 44.9–52.7 | 49.4 ± 1.74 |
| IOD | 27.8 | 24.7–30.6 | 27.8 ± 2.02 | 31.0 | 25.0–31.2 | 23.3–29.5 | 27.8 ± 1.75 | 33.5 | 28.1–34.0 | 31.4 ± 1.42 | 25.2 | 20.4–28.8 | 23.9 ± 1.96 |
| ED | 7.0 | 6.0–9.2 | 7.8 ± 0.96 | 12.1 | 8.2–12.1 | 7.8–9.9 | 9.2 ± 0.87 | 10.2 | 8.6–11.7 | 9.8 ± 0.75 | 8.2 | 7.4–10.3 | 8.7 ± 0.79 |
| NBL | 40.3 | 33.9–52.9 | 40.6 ± 5.50 | 49.4 | 27.6–49.4 | 29.8–36.8 | 35.4 ± 4.80 | 28.4 | 25.9–34.5 | 30.0 ± 2.07 | 27.7 | 22.8–34.0 | 27.4 ± 2.73 |
| MBL | 132.8 | 103.8–161.0 | 124.2 ± 17.71 | 110.3 | 84.6–115.9 | 85.4–116.4 | 99.0 ± 10.11 | 89.8 | 77.2–109.0 | 95.7 ± 9.70 | 83.6 | 76.8–98.5 | 85.4 ± 5.70 |
| IMBL | 44.1 | 34.5–57.7 | 43.5 ± 5.52 | 36.2 | 29.1–42.8 | 30.9–41.9 | 34.1 ± 3.29 | 33.0 | 30.3–40.5 | 34.8 ± 2.63 | 27.0 | 25.7–32.7 | 29.1 ± 2.10 |
| OMBL | 75.3 | 58.8–87.9 | 69.6 ± 9.14 | 64.9 | 42.0–65.2 | 45.6–62.5 | 54.1 ± 6.10 | 54.5 | 46.6–65.6 | 55.5 ± 4.53 | 46.5 | 39.8–51.4 | 46.4 ± 3.14 |
| . | . | . | . | G. longinema(N= 29) . | . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | G. zanaensis(N= 26) . | . | Range . | . | G. fucatus sp. nov.(N= 27) . | G. granosus sp. nov.(N= 24) . | |||||||
| . | Holotype . | Range . | Mean ± SD . | Holotype . | Nujiang (N= 15) . | Lancangjiang (N= 14) . | Mean ± SD . | Holotype . | Range . | Mean ± SD . | Holotype . | Range . | Mean ± SD . |
| SL | 74.6 | 54.7–95.6 | 67.0 | 55.1–95.2 | 64.6–115.1 | 72.9 | 55.0–105.2 | 61.8 | 48.6–117.3 | ||||
| %SL | |||||||||||||
| PDL | 33.2 | 32.5–37.0 | 34.4 ± 1.21 | 37.0 | 32.4–38.7 | 33.6–38.7 | 35.6 ± 1.53 | 34.3 | 32.4–36.8 | 34.2 ± 1.09 | 36.1 | 31.6–37 | 34.7 ± 1.32 |
| PAL | 63.4 | 63.4–68.8 | 66.0 ± 1.44 | 64.9 | 63.7–68.1 | 64.1–68.1 | 66.1 ± 1.24 | 68.9 | 63.3–69.8 | 66.5 ± 1.83 | 63.3 | 59.7–66.3 | 63.3 ± 1.57 |
| PVL | 46.0 | 46.0–51.2 | 49.0 ± 1.41 | 49.0 | 46.4–49.9 | 47.2–51.6 | 48.8 ± 1.35 | 52.1 | 46.2–52.6 | 49.7 ± 1.64 | 46.1 | 44.8–49.7 | 47.5 ± 1.28 |
| PPL | 20.8 | 18.3–23.5 | 20.2 ± 1.41 | 20.6 | 18.2–23.6 | 16.0–22.8 | 19.3 ± 19.1 | 21.4 | 17.2–21.7 | 19.4 ± 1.12 | 17.5 | 17.3–21.2 | 19.1 ± 1.23 |
| LDFB | 11.5 | 10.6–13.7 | 12.2 ± 0.80 | 13.9 | 10.2–13.9 | 11.5–17.9 | 12.6 ± 1.67 | 11.9 | 10.9–14.3 | 12.1 ± 0.73 | 12.6 | 9.5–12.8 | 11.8 ± 0.82 |
| DSL | 16.2 | 12.3–16.8 | 15.2 ± 1.18 | 12.4 | 10.3–13.8 | 11.0–13.1 | 12.4 ± 0.90 | 15.8 | 11.7–16.4 | 14.4 ± 1.47 | 14.2 | 12.1–14.8 | 13.8 ± 0.81 |
| LAFB | 16.2 | 13.0–16.9 | 14.9 ± 0.98 | 14.5 | 12.3–15.9 | 12.9–17.2 | 14.6 ± 1.23 | 14.3 | 13.2–17.9 | 15.6 ± 1.52 | 14.2 | 11.8–15.2 | 13.7 ± 0.85 |
| VFL | 17.7 | 15.0–18.4 | 17.0 ± 0.90 | 17.0 | 14.4–17.1 | 14.9–17.9 | 16.3 ± 0.75 | 16.0 | 13.5–18.5 | 16.0 ± 1.15 | 16.2 | 14.0–18.8 | 16.6 ± 1.08 |
| PFL | 23.9 | 20.9–25.6 | 23.5 ± 1.23 | 22.5 | 20.0–26.1 | 20.2–25.1 | 22.9 ± 1.58 | 22.5 | 19.0–24.3 | 21.9 ± 1.59 | 25.4 | 22.8–26.5 | 24.5 ± 0.85 |
| PSL | 18.2 | 15.8–20.6 | 17.6 ± 1.32 | 17.5 | 13.0–18.8 | 13.9–19.6 | 16.8 ± 1.54 | 17.7 | 14.9–19.0 | 16.9 ± 1.23 | 18.0 | 16.5–19.7 | 18.1 ± 0.75 |
| CFL | 23.9 | 20.2–25.9 | 22.6 ± 1.37 | 23.9 | 19.3–25.6 | 20.2–23.7 | 22.3 ± 1.51 | 25.4 | 18.6–25.9 | 22.0 ± 1.82 | 24.3 | 20.5–25.9 | 23.5 ± 1.17 |
| LAdFB | 18.6 | 14.1–19.6 | 16.9 ± 1.46 | 16.0 | 13.1–17.8 | 13.5–17.4 | 15.3 ± 1.12 | 13.3 | 12.8–16.7 | 15.2 ± 1.15 | 14.9 | 12.8–15.9 | 14.4 ± 0.85 |
| DAD | 23.9 | 19.8–26.7 | 23.4 ± 2.08 | 23.4 | 20.3–26.2 | 21.8–26.2 | 23.0 ± 1.49 | 26.2 | 19.5–26.6 | 23.0 ± 1.98 | 20.9 | 16.7–23.6 | 20.7 ± 1.59 |
| PAD | 14.3 | 12.0–16.4 | 14.2 ± 1.21 | 14.6 | 14.2–18.0 | 14.3–18.2 | 15.9 ± 1.26 | 15.9 | 13.5–18.7 | 16.3 ± 1.33 | 17.5 | 15.7–21.6 | 19.2 ± 1.44 |
| LCP | 16.8 | 14.8–18.9 | 17.1 ± 1.16 | 17.6 | 17.3–20.7 | 16.8–21.9 | 18.7 ± 1.32 | 16.5 | 16.0–20.4 | 18.0 ± 1.33 | 20.1 | 18.2–23.7 | 21.4 ± 1.47 |
| DCP | 6.4 | 5.5–7.9 | 6.7 ± 0.50 | 8.4 | 7.1–10.6 | 7.4–10.1 | 8.9 ± 0.88 | 9.1 | 8.2–11.1 | 9.3 ± 0.88 | 7.0 | 5.7–7.6 | 6.7 ± 0.44 |
| BDA | 12.2 | 11.4–15.8 | 13.4 ± 1.32 | 17.3 | 14.6–20.4 | 15.9–19.9 | 17.2 ± 1.33 | 16.9 | 15.5–21.1 | 17.4 ± 1.28 | 13.9 | 12.9–16.5 | 14.1 ± 0.76 |
| HL | 24.9 | 23.3–26.7 | 24.8 ± 0.91 | 26.0 | 22.7–27.0 | 23.8–27.7 | 25.2 ± 1.17 | 24.1 | 21.7–25.6 | 24.1 ± 0.93 | 25.7 | 23.1–26.0 | 24.6 ± 0.80 |
| HW | 17.8 | 17.5–21.5 | 19.2 ± 1.09 | 20.9 | 19.4–22.8 | 19.3–22.9 | 20.7 ± 0.93 | 21.4 | 18.1–21.8 | 20.3 ± 0.94 | 19.1 | 16.6–19.5 | 18.1 ± 0.75 |
| HD | 12.7 | 12.3–16.7 | 14.6 ± 1.36 | 17.9 | 13.6–17.9 | 14.5–18.1 | 15.8 ± 1.32 | 16.0 | 14.4–17.4 | 16.0 ± 0.95 | 16.0 | 13.1–16.2 | 14.8 ± 0.94 |
| AAL | 14.2 | 11.3–15.2 | 13.7 ± 0.85 | 13.6 | 12.6–15.4 | 12.7–16.0 | 14.2 ± 1.04 | 14.7 | 11.9–15.7 | 13.9 ± 0.93 | 16.3 | 14.4–18.0 | 16.0 ± 0.89 |
| AAW | 9.5 | 7.9–11.4 | 9.5 ± 0.80 | 10.6 | 9.2–11.6 | 9.2–12.3 | 10.7 ± 0.78 | 10.0 | 8.9–11.4 | 10.2 ± 0.66 | 10.2 | 8.7–10.3 | 9.5 ± 0.51 |
| %HL | |||||||||||||
| SnL | 44.6 | 44.6–49.8 | 47.6 ± 1.41 | 49.4 | 45.8–52.4 | 44.1–54.0 | 49.7 ± 2.36 | 53.4 | 44.0–53.4 | 49.1 ± 2.43 | 49.7 | 44.9–52.7 | 49.4 ± 1.74 |
| IOD | 27.8 | 24.7–30.6 | 27.8 ± 2.02 | 31.0 | 25.0–31.2 | 23.3–29.5 | 27.8 ± 1.75 | 33.5 | 28.1–34.0 | 31.4 ± 1.42 | 25.2 | 20.4–28.8 | 23.9 ± 1.96 |
| ED | 7.0 | 6.0–9.2 | 7.8 ± 0.96 | 12.1 | 8.2–12.1 | 7.8–9.9 | 9.2 ± 0.87 | 10.2 | 8.6–11.7 | 9.8 ± 0.75 | 8.2 | 7.4–10.3 | 8.7 ± 0.79 |
| NBL | 40.3 | 33.9–52.9 | 40.6 ± 5.50 | 49.4 | 27.6–49.4 | 29.8–36.8 | 35.4 ± 4.80 | 28.4 | 25.9–34.5 | 30.0 ± 2.07 | 27.7 | 22.8–34.0 | 27.4 ± 2.73 |
| MBL | 132.8 | 103.8–161.0 | 124.2 ± 17.71 | 110.3 | 84.6–115.9 | 85.4–116.4 | 99.0 ± 10.11 | 89.8 | 77.2–109.0 | 95.7 ± 9.70 | 83.6 | 76.8–98.5 | 85.4 ± 5.70 |
| IMBL | 44.1 | 34.5–57.7 | 43.5 ± 5.52 | 36.2 | 29.1–42.8 | 30.9–41.9 | 34.1 ± 3.29 | 33.0 | 30.3–40.5 | 34.8 ± 2.63 | 27.0 | 25.7–32.7 | 29.1 ± 2.10 |
| OMBL | 75.3 | 58.8–87.9 | 69.6 ± 9.14 | 64.9 | 42.0–65.2 | 45.6–62.5 | 54.1 ± 6.10 | 54.5 | 46.6–65.6 | 55.5 ± 4.53 | 46.5 | 39.8–51.4 | 46.4 ± 3.14 |
SL, standard length; PDL, predorsal length; PAL, preanal length; PVL, prepelvic length; PPL, prepectoral length; LDFB, length of dorsal-fin base; DSL, dorsal-spine length; LAFB, length of anal-fin base; VFL, pelvic-fin length; PFL, pectoral-fin length; PSL, pectoral-spine length; CFL, caudal-fin length; LAdFB, length of adipose-fin base; DAD, dorsal to adipose distance; PAD, post-adipose distance; LCP, length of caudal peduncle; DCP, depth of caudal peduncle; BDA, body depth at anus; HL, head length; HW, head width; HD, head depth; AAL, adhesive apparatus length; AAW, adhesive apparatus width; SnL, snout length; IOD, interorbital distance; ED, eye diameter; NBL, nasal barbel length; MBL, maxillary barbel length; IMBL, inner mandibular barbel length; OMBL, outer mandibular barbel length.
Head depressed, body subcylindrical. Dorsal profile rising evenly from tip of snout to origin of dorsal fin, and then sloping gently ventrally from origin of dorsal fin to end of caudal peduncle. Ventral profile flat to anal-fin base, then sloping gently dorsally from anal-fin base to end of caudal peduncle. Anus and urogenital openings located at vertical through middle of adpressed pelvic fin. Skin tuberculate, with tubercles uniformly arranged on sides of body; tubercles particularly distinct on dorsal surface of neurocranium. Lateral line complete and midlateral, laterosensory pores rimmed in beige. Anterior nuchal plate element triangular, with straight anterolateral margins and without extensive contact with posterior nuchal plate element. Vertebrae 17 + 22 = 39 (1) or 16 + 22 = 38 (1).
Head depressed and broad, triangular when viewed laterally. Snout gently convex when viewed from above. Anterior and posterior nares separated only by base of nasal barbel. Eyes small and ovoid, horizontal axis longest, located on dorsal half of head. Gill openings broad, extending from directly beneath post-temporal to isthmus.
Barbels in four pairs. Maxillary barbel thick, extending beyond base of last pectoral-fin ray. Nasal barbel slender, extending beyond posterior orbital margin. Inner mandibular barbel extending to middle of thoracic adhesive apparatus. Outer mandibular barbel originating posterolateral of inner mandibular barbel, extending to middle of pectoral-fin base.
Mouth inferior, premaxillary tooth band partially exposed when mouth closed. Oral teeth small and villiform, in irregular rows on all tooth-bearing surfaces. Premaxillary teeth in single crescentic-shaped band. Dentary teeth in two patches separated by narrow gap at midline. Palate edentate.
Dorsal fin nearer to snout-tip than to adipose-fin origin, with I,6 rays; dorsal margin of fin truncate; anterior margin of spine smooth, posterior margin with weak serrations. Adipose fin straight anteriorly and angular posteriorly. Caudal fin deeply forked, lobes almost equal, with i,7,7,i (2) or i,7,8,i (23) principal rays. Procurrent rays symmetrical, extending only slightly anterior to fin base and consisting of four to six rays. Anal-fin origin slightly anterior to vertical through adipose-fin origin. Anal fin with straight anterior margin and straight or slightly concave posterior margin; with ii,9 (19) or ii,10 (7) rays. Pectoral fin with I,9 (11) or I,10 (15) rays; posterior fin margin straight; anterior margin of spine smooth, posterior margin with nine to 13 serrations. Pelvic-fin origin at vertical through posterior end of dorsal-fin base. Pelvic fin with slightly convex anterior margin and i,5 rays; tip of adpressed fin not reaching anal-fin origin.
Thoracic adhesive apparatus present, consisting of skin ridges (striae) in an elongate oval field extending from isthmus to middle of pectoral-fin base and with narrow median depression on posterior third. Striae adjacent to median depression anastomosing to form a diverging pattern running along edges of depression.
Coloration:
In 75% ethanol: dorsal and lateral surfaces of head and body brown, fading to greyish yellow on long-term preservation. Ventral surfaces of head and body greyish beige. Body without distinct pale blotches; indistinct patches sometimes present on infraorbital space, cranial fontanel, and nuchal plate elements. Lateral line evident as narrow, pale stripe. Base of all fins dark brown, fading to yellow or hyaline distally. Fin rays with scattered melanophores; fin membranes hyaline. Maxillary and nasal barbels brown dorsally, light yellow ventrally.
Distribution:
Known from upper reaches of the Salween River (Nujiang) drainage (Fig. 7).
Distributions of the four species studied; ▴, Glyptothorax zanaensis; ◆, Glyptothorax granosus sp. nov. ;  , Glyptothorax longinema; ●, Glyptothorax fucatus sp. nov.
, Glyptothorax longinema; ●, Glyptothorax fucatus sp. nov.
GLYPTOTHORAX LONGINEMALI, 1984(FIG. 8)
Glyptothorax longinema
Li, 1984:81, fig. 6(type locality: Liuku, Yunnan, Nujiang drainage).
Glyptothorax longinema. A, KIZ 740197, holotype, 67 mm SL; China: Bijiang; B, KIZ 2000000559, 60.1 mm SL; China: Shangjiang; C, KIZ 749356, holotype of Glyptothorax rubermentus, 90.3 mm SL; China: Wayao. a, dorsal view; b, lateral view; c, ventral view. Scale bars = 10 mm.
Glyptothorax rubermentus
Li, 1984:83, fig. 8(type locality: Baoshan, Yunnan, Lancangjiang drainage).
Glyptothorax zanaensis
Wu & Wu, 1992:532, fig. 146.
Glyptothorax zainaensis (non Wu, He & Chu)
Chu, Mo & Kuang, 1990,:187 (in part); Yang, 1998: 298, fig. 217; Chu & Mo, 1999: 146 (in part).
Material examined:
Nujiang drainage: KIZ 741097 (holotype), 67.0 mm SL; KIZ 740198–740200 (three paratypes), 55.1–58.9 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, Liuku Township, Bijiang. KIZ 1981001381–1981001383 (3), 82.3–89.1 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County. KIZ 2006000865 (1), 87.8 mm SL; KIZ 2006000870 (1), 95.2 mm SL; KIZ 2006000878 (1), 89.0 mm SL; China: Yunnan, Baoshan Prefecture, Longlin County, Sanjiangkou. KIZ 2002002337–2002002341 (5), 71.8–91.0 mm SL; China: Yunnan, Lincang Prefecture, Yongde County, Nanting River. CAS 223290 (1), 75.6 mm SL; CAS 229924 (6), 37.8–67.9 mm SL; China: Yunnan, Mangyin River, from mouth upstream c. 250 m to point c. 100 m downstream of bridge, 24°24'55.4''N, 98°58'35.3''E. CAS 226099 (5), 58.3–72.7 mm SL; China: Yunnan, Baoshan Prefecture, Longling County, Gongyang River from mouth at confluence of Nujiang (Salween River) upstream c. 400 m, 24°10'41.1'N, 98°54'12.5'E. CAS 223055 (3), 36.4–39.2 mm SL; China: Yunnan, Baoshan Prefecture, Longling County, Taozhai River from its mouth at Nujiang (Salween River) to a point c. 300 m upstream from road bridge, 24°13''N, 99°1''E. ZRC 50712 (4), 77.2–94.6 mm SL; China: Yunnan, Nujiang at Daojie, west of Baoshan, 25°0''N, 98°53''E.
Lancangjiang drainage: KIZ 749356 (holotype of G. rubermentus), 90.3 mm SL; KIZ 749357–749359 (three paratypes of G. rubermentus), 64–86 mm SL; China: Yunnan, Baoshan Prefecture, Wayao Township. KIZ 2005000592 (1), 115.1 mm SL; China: Yunnan, Diqin Prefecture, Deqin County, Gushui. KIZ 74884 (1), 67.7 mm SL; China: Yunnan, Diqin Prefecture, Weixi County, Baijixun. KIZ 1977001502 (1), 71.2 mm SL; KIZ 1977001505–1977001506 (3), 64.6–73 mm SL; China: Yunnan, Nujiang Prefecture, Lanping County. KIZ 1978000845 (1), 94.5 mm SL; KIZ 1978000847 (1), 79.5 mm SL; KIZ 1978000849 (1), 90.8 mm SL; China: Yunnan, Lincang Prefecture, Yun County. KIZ 1976003603–1976003604 (2), 73.5–110 mm SL; China: Yunnan, Dali Prefecture, Yangbi County.
Diagnosis:
Glyptothorax longinema can be distinguished from other congeners in the upper Irrawaddy, Mekong, and Salween River drainages with a uniformly dark-coloured body in having a thoracic adhesive apparatus consisting of striae arranged in a broad oval field (in a somewhat cardiform shape vs. a more elongate, ovoid field in other congeners; Fig. 5). It further differs from G. burmanicus in having the depressed area in the thoracic adhesive apparatus not wholly enclosed by ridges (vs. ridges of the thoracic adhesive apparatus enclosing an ovoid depressed region in the centre) and a shorter head (22.7–27.7% SL vs. 27.6–30.8); from G. deqinensis in having more serrations on the posterior edge of the pectoral spine (ten to 12 vs. five to eight); from G. fucatus in having a uniformly coloured lateral surface of the body (vs. becoming paler ventrally immediately under lateral line) and a triangular anterior nuchal plate element without (vs. with) extensive contact with the posterior nuchal plate element (Fig. 6); and from G. granosus in having a triangular anterior nuchal plate element without (vs. with) extensive contact with the posterior nuchal plate element (Fig. 7), a deeper caudal peduncle (7.1–10.6% SL vs. 5.7–7.6), and a wider head (19.3–22.9% SL vs. 16.6–19.5). Glyptothorax longinema is further distinguished from G. longicauda in having shorter post-adipose distance (14.2–18.2% SL vs. 18.2–23.0) and longer nasal barbel (27.6–49.4% HL vs. 16.1–23.8), from G. longjiangensis in having small, conical tubercles (vs. large plaques bearing unculiferous ridges) on the dorsal surface of the head and longer nasal barbel (27.6–49.4% HL vs. 18.1–27.9), from G. macromaculatus in having distally expanded (vs. pointed) neural spines, without (vs. with) a diverging pattern of striae running along the edges of the median depression in the thoracic adhesive apparatus and a shorter head (22.7–27.7% SL vs. 27.4–34.4), and from G. minimaculatus in lacking (vs. having) dark spots on the body and longer nasal barbel (27.6–49.4% HL vs. 18.3–26.2). It further differs from G. ngapang in having longer adipose-fin base (13.1–17.8% SL vs. 10.7–14.0), deeper caudal peduncle (7.1–10.6% SL vs. 5.3–7.4), and wider head (19.3–22.9% SL vs. 15.8–19.7), from G. obliquimaculatus in lacking (vs. having) both the ridges of the thoracic adhesive apparatus extending onto the gular region and irregular dark blotches on the flanks and having a longer adipose-fin base (13.1–17.8% SL vs. 8.6–12.9), and from G. trilineatus in lacking (vs. having) a distinct pale midlateral line on the flank, and from G. zanaensis in lacking (vs. having) a diverging pattern of striae running along the edges of the median depression in the thoracic adhesive apparatus and having a deeper body (depth at anus 14.6–20.4% SL vs. 11.4–15.8) and caudal peduncle (7.1–10.6% SL vs. 5.5–7.9).
Description:
Biometric data in Table 4.
Head depressed, body subcylindrical. Dorsal profile rising evenly from tip of snout to origin of dorsal fin, and then sloping gently ventrally from origin of dorsal fin to end of caudal peduncle. Ventral profile flat to anal-fin base, then sloping gently dorsally from anal-fin base to end of caudal peduncle. Anus and urogenital openings located at vertical through middle of adpressed pelvic fin. Skin tuberculate, uniformly arranged on sides of body; tubercles particularly distinct along lateral line and in opercular region. Lateral line complete and midlateral, laterosensory pores rimmed in beige. Anterior nuchal plate element triangular, with straight anterolateral margins and without extensive contact with posterior nuchal plate element. Vertebrae 16 + 22 = 38 (3), 16 + 23 = 39 (2), or 17 + 22 = 39 (1).
Head depressed and broad, triangular when viewed laterally. Snout gently convex when viewed from above. Anterior and posterior nares separated only by base of nasal barbel. Eyes small and ovoid, horizontal axis longest, located on dorsal half of head. Gill openings broad, extending from directly beneath post-temporal to isthmus.
Barbels in four pairs. Maxillary barbel long and thick, extending beyond base of last pectoral-fin ray. Nasal barbel slender, extending to middle of orbit. Inner mandibular barbel extending to middle of thoracic adhesive apparatus. Outer mandibular barbel originating posterolateral of inner mandibular barbel, extending to middle of pectoral-fin base.
Mouth inferior, premaxillary tooth band partially exposed when mouth closed. Oral teeth small and villiform, in irregular rows on all tooth-bearing surfaces. Premaxillary teeth in single crescentic-shaped band. Dentary teeth in two patches separated by narrow gap at midline. Palate edentate.
Dorsal fin slightly further from snout-tip than to adipose-fin origin, with I,6 rays; dorsal margin of fin truncate; anterior margin of spine smooth, posterior margin with weak serrations. Adipose fin straight anteriorly and angular posteriorly. Caudal fin deeply forked, lower lobe slightly longer than upper lobe and with i,7,8,i principal rays. Procurrent rays symmetrical, extending only slightly anterior to fin base and consisting of four to seven rays. Anal-fin origin slightly anterior to vertical through adipose-fin origin. Anal fin with straight anterior margin and straight or slightly concave posterior margin; with ii,8 (5), ii,9 (11), or ii,10 (13) rays. Pectoral fin with I,9 (11) or I,10 (18) rays; posterior fin margin straight; anterior margin of spine smooth, posterior margin with ten to 12 serrations. Pelvic-fin origin posterior to vertical through posterior end of dorsal-fin base. Pelvic fin with slightly convex margin and i,5 rays; tip of adpressed fin not reaching anal-fin origin.
Thoracic adhesive apparatus present, consisting of skin ridges (striae) in an elongate oval field extending from isthmus to posterior half of pectoral-fin base and with median depression present on posterior one-third to half. Striae orientated anterodistally, radiating from median depression.
Coloration:
In 75% ethanol: dorsal and lateral surfaces of head and body brown, fading to greyish yellow on long-term preservation. Ventral surfaces of head and body greyish beige. Dorsal surface of neurocranium with indistinct pale blotches; nuchal plate elements distinctly pale. A faint, thin, light grey mid-dorsal stripe outlining distal tips of neural spines extending from base of last dorsal-fin ray to adipose-fin origin. Laterosensory pores along lateral line rimmed in beige, imparting appearance of a narrow pale line. Dorsal fin and anal fin with brown bases and dark brown bands on middle third of fins, rest of fin yellow to hyaline. Pectoral and pelvic fins with brown bases and dark brown bands on middle third of fins dorsally, light yellow ventrally. Adipose fin yellow at base and distally, remainder of fin dark brown. Caudal fin brown, with tips of lobes hyaline. Maxillary and nasal barbels brown dorsally, light yellow ventrally.
Distribution:
Known from Salween (Nujiang) and Mekong (Lancangjiang) River drainages (Fig. 7).
Remarks:
As G. longinema and G. rubermentus are simultaneous subjective synonyms, we use our prerogative as first revisers by designating the priority of G. longinema over G. rubermentus in accordance with Article 24.2 of the International Code of Zoological Nomenclature (ICZN, 1999).
GLYPTOTHORAX GRANOSUSSP. NOV. (FIG. 9)
Material examined:
Holotype: KIZ 2000000586 (1), 61.8 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, Liuku Township, Manbu Village, Manbu River (a tributary of Nujiang), 25°52'44.4''N, 98°50'27.8''E, D. Catania, X. Y. Chen, Z. M. Chen, 22–24.vii.2000.
Glyptothorax granosus, KIZ 2000000534, holotype, 61.8 mm SL, China: Manbu River; A, dorsal view; B, lateral view; C, ventral view. Scale bar = 10 mm.
Paratypes: KIZ 2000000335 (one juvenile), 42.3 mm SL; KIZ 2000000543 (1), 52.7 mm SL; KIZ 2000000548 (1), 68.7 mm SL; KIZ 2000000552 (1), 72.8 mm SL; KIZ 2000000554–2000000555 (2), 48.6–65.9 mm SL; KIZ 2000000557 (1), 69 mm SL; KIZ 2000000560 (1), 68.7 mm SL; KIZ 2000000574 (1), 61.6 mm SL; KIZ 2000000576 (1), 72.6 mm SL; KIZ 2000000584–2000000585 (2), 61.8–67.2 mm SL; data as for holotype. CAS 222110 [2 alcohol preserved (alc.), 1 cleared and stained skeleton (c&s)], 47.0–67.3 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, west bank of Nujiang (Salween River), 11.5 km south of Liuku on road to Shangjiang, 25°45'26.5''N, 98°51'56.6''E; C. J. Ferraris et al., 29.vii.2000. CAS 223290 (4), 69.7–80.7 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, Mabu River, 3.9 km north of Liuku on road to Fugong, west bank of Nujiang (Salween River), and in Nujiang just south of Mabu River mouth, 25°52'44.4''N, 98°50'27.8''E; D. Catania et al., 22–24.vii.2000. CAS 223324 (5), 69.7–77.2 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, west bank of Nujiang (Salween River), 52.2 km north of Liuku (and 1–2 km south of Chengan) on road to Fugong, 26°14'32.6''N, 98°52'05.6''E; C. J. Ferraris et al., 28.vii.2000. CAS 223338 (2), 71.2–72.0 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, west bank of Nujiang (Salween River), 80 km north of Liuku on road to Fugong, 26°27'42.2'N, 98°53'52.2'E; C. J. Ferraris et al., 20.vii.2000. CAS 223346 (2), 74.9–78.0 mm SL; China: Yunnan, Nujiang Prefecture, Gongshan County, Nujiang (Salween River) just below Yuange stream on east bank, c. 14 km south of bridge at Gongshan, 27°38'5.8''N, 98°43'51.2''E; C. J. Ferraris et al., 11.vii.2000. CAS 226795 (5), 73.0–95.7 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, Dashaba, 25°58'22.8''N, 98°50'31.5''E; local fishermen, 24.iv.2004.
Additional nontype material: KIZ 2003006175 (1), 72.8 mm SL; KIZ 2003006179–2003006186 (8), 40.5–81.9 mm SL; KIZ 2003006308–2003006310 (3), 50.4–53.7 mm SL; KIZ 2003006312 (1), 83.8 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, Liuku Township, Yuejinqiao. KIZ 2003003112 (1), 84.7 mm SL; KIZ 2003003124 (1), 80.2 mm SL; KIZ 2003003126 (1), 72.4 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, Liuku Township, Xiangyangqiao. KIZ 2004014151 (1), 79.3 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, Liuku Township, Hongqiqiao. KIZ 05223–05226 (4), 97.4–117.3 mm SL; China: Yunnan, Nujiang Prefecture, Lushui County, Daxingdi. KIZ 2000000539–2000000540 (2), 54.5–78.4 mm SL; China: Yunnan, Nujiang Prefecture, Fugong County, Zilijia Township. KIZ 2000000521 (1), China: Yunnan, Nujiang Prefecture, Gongshan County, Puladi Township.
Diagnosis:
Glyptothorax granosus sp. nov. can be distinguished from G. burmanicus in having the depressed area in the thoracic adhesive apparatus not wholly enclosed by ridges (vs. ridges of the thoracic adhesive apparatus enclosing an ovoid depressed region in the centre), a longer caudal peduncle (18.2–23.7% SL vs. 14.8–18.7), and a shorter (23.1–26.0% SL vs. 27.6–30.8) and narrower head (16.6–19.5% SL vs. 20.0–25.7); from G. deqinensis in having more serrations on the posterior edge of the pectoral spine (nine to 13 vs. five to eight), narrower head (16.6–19.5 % SL vs. 19.5–21.7), and shorter inner and outer mandibular barbels (inner mandibular barbel: 25.7–32.7% HL vs. 37.2–57.4; outer mandibular barbel: 39.8–51.4% HL vs. 59.2–85.7); from G. fucatus in having a uniformly coloured lateral surface of the body (vs. lateral surface of the body ventrally becoming paler immediately under lateral line), a triangular anterior nuchal plate element with (vs. without) saddle-shaped lateral expansions of the pterygiophores (Fig. 6), a more slender body (depth at anus 12.9–16.5% SL vs. 15.5–21.1) and caudal peduncle (5.7–7.6% SL vs. 8.2–11.1), and smaller interorbital distance (20.4–28.8% HL vs. 28.1–34.0); and from G. longicauda in having fewer vertebrae (37–39 vs. 39–42). It differs from G. longinema in having a more slender caudal peduncle (5.7–7.6% SL vs. 7.1–10.6), narrower head (16.6–19.5% SL vs. 19.3–22.9), and a triangular anterior nuchal plate element with (vs. without) saddle-shaped lateral expansions of the pterygiophores (Fig. 6), from G. longjiangensis in having small, conical tubercles (vs. large plaques bearing unculiferous ridges) on the dorsal surface of the head, from G. macromaculatus in having a triangular anterior nuchal plate element with (vs. without) saddle-shaped lateral expansions of the pterygiophores, distally expanded (vs. pointed) neural spines, without (vs. with) a diverging pattern of striae running along the edges of the median depression in the thoracic adhesive apparatus, and a shorter (23.1–26.0% SL vs.27.4–34.4) and narrower head (16.6–19.5% SL vs. 20.6–25.4), and from G. minimaculatus in lacking (vs. with) dark spots on the body and having a triangular anterior nuchal plate element with (vs. without) saddle-shaped lateral expansions of the pterygiophores. Glyptothorax granosus is distinguished from G. ngapang in having longer nasal barbel (22.8–34.0% HL vs. 14.1–23.5), from G. obliquimaculatus in lacking (vs. having) both the ridges of the thoracic adhesive apparatus extending onto the gular region and irregular dark blotches on the flanks and having a longer adipose-fin base (12.8–15.9% SL vs. 8.6–12.9), a more slender caudal peduncle (5.7–7.6% SL vs. 8.6–9.8), and a narrower head (16.6–19.5% SL vs. 19.1–24.0), from G. trilineatus in lacking (vs. having) a distinct pale midlateral line on the flank, and from G. zanaensis in having a longer thoracic adhesive apparatus (14.4–18.0% SL vs. 11.3–15.2), post-adipose distance (15.7–21.6% SL vs. 12.0–16.4), and caudal peduncle (18.2–23.7% SL vs. 14.8–18.9), shorter barbels (length of nasal barbel 22.8–34.0% HL vs. 33.9–52.9; length of maxillary barbel 76.8–98.5% HL vs. 103.8–161.0; length of inner mandibular barbel 25.7–32.7% HL vs. 34.5–57.7; length of outer mandibular barbel 39.8–51.4% HL vs. 58.8–87.9), a triangular anterior nuchal plate element with (vs. without) saddle-shaped lateral expansions of the pterygiophores (Fig. 6), and without (vs. with) a diverging pattern of striae running along the edges of the median depression in the thoracic adhesive apparatus (Fig. 5).
Description:
Biometric data in Table 4.
Head depressed, body subcylindrical. Dorsal profile rising evenly from tip of snout to origin of dorsal fin, and then sloping gently ventrally from origin of dorsal fin to end of caudal peduncle. Ventral profile flat to anal-fin base, then sloping gently dorsally from anal-fin base to end of caudal peduncle. Anus and urogenital openings located at vertical through middle of adpressed pelvic fin. Skin tuberculate, with tubercles densely and uniformly arranged on sides of body and dorsal surface of neurocranium. Juveniles with tubercles prominently arranged in longitudinal rows on flanks. Lateral line complete and midlateral, laterosensory pores rimmed in beige, frequently with branches of laterosensory canal system visibly radiating from it. Anterior nuchal plate element triangular, with saddle-shaped lateral extensions and extensive contact with posterior nuchal plate element. Vertebrae 16 + 21 = 37 (2), 16 + 22 = 38 (10), 16 + 23 = 39 (1), or 17 + 22 = 39 (1).
Head depressed and broad, triangular when viewed laterally. Snout acutely convex when viewed from above. Anterior and posterior nares separated only by base of nasal barbel. Eyes small and ovoid, horizontal axis longest, located on dorsal half of head. Gill openings broad, extending from directly beneath post-temporal to isthmus.
Barbels in four pairs. Maxillary barbel thick, extending to base of last pectoral-fin ray. Nasal barbel slender, extending to anterior orbital margin. Inner mandibular barbel extending to anterior edge of thoracic adhesive apparatus. Outer mandibular barbel originating posterolateral of inner mandibular barbel, extending to base of pectoral-fin spine.
Mouth inferior, premaxillary tooth band partially exposed when mouth closed. Oral teeth small and villiform, in irregular rows on all tooth-bearing surfaces. Premaxillary teeth in single crescentic-shaped band. Dentary teeth in two patches separated by narrow gap at midline. Palate edentate.
Dorsal fin equidistant from snout tip to adipose-fin origin, with I,6 rays; dorsal margin truncate; anterior margin of spine smooth, posterior margin with weak serrations. Adipose fin straight anteriorly and angular posteriorly. Caudal fin deeply forked, lobes almost equal, with i,7,7,i (8) or i,7,8,i (16) principal rays. Procurrent rays symmetrical, extending only slightly anterior to fin base and consisting of five to seven rays. Anal-fin origin slightly anterior to vertical through adipose-fin origin. Anal fin with straight anterior margin and straight or slightly concave posterior margin; with ii,9 (18) or ii,10 (6) rays. Pectoral fin with I,9 (4), I,10 (18), or I,11 (2) rays; posterior fin margin straight; anterior spine margin smooth, posterior spine margin with nine to 13 serrations. Pelvic-fin origin at vertical through posterior end of dorsal-fin base. Pelvic fin with slightly convex margin and i,5 rays; tip of adpressed fin not reaching anal-fin origin.
Thoracic adhesive apparatus present, consisting of narrow skin ridges (striae) in an elongate oval field extending from isthmus to end of pectoral-fin base and with median depression present on posterior one-third to half. Striae orientated anterodistally, radiating from median depression.
Coloration:
In 75% ethanol: dorsal and lateral surfaces of head and body brown. Ventral surface of head and body greyish beige. Dorsal surface of head with distinct pale patches; nuchal plate elements distinctly pale. A faint, thin, light grey mid-dorsal stripe outlining distal tips of neural spines extending posterior to base of last dorsal-fin ray to adipose-fin origin. Laterosensory pores along lateral line rimmed in beige tubercles, imparting appearance of a narrow pale line; branches of canals sometimes imparting a spine-like form, or herringbone pattern. Dorsal and anal fins, and dorsal surfaces of pectoral and pelvic fins with dark brown bases and bands on middle third, rest of fins yellow to hyaline. Adipose fin yellow at base and distally, remainder of fin dark brown. Caudal fin brown, with tips of lobes hyaline. Maxillary and nasal barbels brown dorsally, light yellow ventrally.
Distribution:
Known from the upper Salween River (Nujiang) drainage (Fig. 7)
Etymology:
The specific epithet granosus is Latin, meaning ‘full of grain’, in allusion to the appearance suggested by the prominent tubercles in the juveniles of this species. Used as an adjective.
GLYPTOTHORAX FUCATUSSP. NOV. (FIG. 10)
Material examined:
Holotype: KIZ 20050410936 (1), 72.9 mm SL; China: Yunnan, Lincang Prefecture, Cangyuan County, Banhong Township, Fugong Village, Fugong River (a tributary of Xiaohei River, which is a tributary of Nanting River, which is itself a tributary to the Nujiang), 23°19'47.2''N, 99°07'28.3''E, about 963 m asl.; X. Y. Chen, X. F. Pan, G. H. Yu and Z. S. Wang; 10.iv.2005.
Paratypes: KIZ 20050410922–20050410931 (10), 82.1–103.2 mm SL; KIZ 20050410934 (1), 75.8 mm SL; KIZ 20050410937–941 (5), 61.5–75.7 mm SL; KIZ 20050410943 (1), 63.8 mm SL; KIZ 20050410947 (1), 55.0 mm SL; data as for holotype.
Additional nontype materials: KIZ 200504111477–200504111484 (8), 64.3–88.3 mm SL; China: Yunnan, Lincang Prefecture, Cangyuan County, Banlao Township, Mengnong River (a tributary of Nangun River, which is itself part of the Salween drainage).
Glyptothorax fucatus, KIZ 20050410936, holotype, 72.9 mm SL, China: Fugong River; A, dorsal view; B, lateral view; C, ventral view. Scale bar = 10 mm.
Diagnosis:
Glyptothorax fucatus sp. nov. can be distinguished from other congeners in the upper Irrawaddy, Mekong, and Salween River drainages with the lateral surface of the body ventrally becoming paler immediately below lateral line (vs. lateral surface uniformly coloured, with vertical maculates, or with dark spots). It further differs from G. burmanicus in having the depressed area in the thoracic adhesive apparatus not wholly enclosed by ridges (vs. ridges of the thoracic adhesive apparatus enclosing an ovoid depressed region in the centre), a deeper caudal peduncle (8.2–11.1% SL vs. 6.5–8.7), shorter head (21.7–25.6% SL vs. 27.6–30.8), and larger eye (diameter 8.6–11.7% HL vs. 5.5–9.1); from G. deqinensis in having more serrations on the posterior edge of the pectoral spine (nine to 12 vs. five to eight) and a deeper body (depth at anus 15.5–21.1% SL vs. 13.5–15.8) and caudal peduncle (8.2–11.1% SL vs. 6.7–8.5); from G. granosus in having a triangular anterior nuchal plate element without (vs. with) saddle-shaped lateral expansions of the pterygiophores (Fig. 6), a deeper body (depth at anus 15.5–21.1% SL vs. 12.9–16.5) and caudal peduncle (8.2–11.1% SL vs. 5.7–7.6) and a larger interorbital distance (28.1–34.0% SL vs. 20.4–28.8); and from G. longicauda in having a shorter postadipose distance (13.5–18.7% SL vs. 18.2–23.0), and deeper body (depth at anus 15.5–21.1% SL vs. 11.0–16.9) and caudal peduncle (8.2–11.1% SL vs. 5.7–8.4). It differs from G. longinema in having a triangular anterior nuchal plate element with concave (vs. straight) anterolateral edges with (vs. without) extensive contact with the posterior nuchal plate element (Fig. 6), from G. longjiangensis in having small, conical tubercles (vs. large plaques bearing unculiferous ridges) on the dorsal surface of the head, and deeper caudal peduncle (8.2–11.1% SL vs. 6.9–8.8), from G. macromaculatus in having distally expanded (vs. pointed) neural spines, without (vs. with) a diverging pattern of striae running along the edges of the median depression in the thoracic adhesive apparatus, and shorter head (21.7–25.6% SL vs. 27.4–34.4), and from G. minimaculatus in lacking (vs. having) dark spots on the body, having a triangular anterior nuchal plate element with concave (vs. straight) anterolateral edges with (vs. without) extensive contact with the posterior nuchal plate element, and a deeper caudal peduncle (8.2–11.1% SL vs. 7.4–7.9). Glyptothorax fucatus is distinguished from G. ngapang in having a deeper body (depth at anus 15.5–21.1% SL vs. 11.2–16.4) and caudal peduncle (8.2–11.1% SL vs. 5.3–7.4), from G. obliquimaculatus in lacking (vs. having) both the ridges of the thoracic adhesive apparatus extending onto the gular region and irregular dark blotches on the flanks and having a longer adipose-fin base (12.8–16.7% SL vs. 8.6–12.9), from G. trilineatus in lacking (vs. with) a distinct pale midlateral line on the flank, and having a deeper body (depth at anus 15.5–21.1% SL vs. 10.1–16.2), and from G. zanaensis in having a triangular anterior nuchal plate element with concave (vs. straight) anterolateral edges with (vs. without) extensive contact with the posterior nuchal plate element (Fig. 6), deeper body (depth at anus 15.5–21.1% SL vs. 11.4–15.8) and caudal peduncle (8.2–11.1% SL vs. 5.5–7.9), larger eye (diameter 8.6–11.7% HL vs. 6.0–9.2), and shorter nasal and maxillary barbels (length of nasal barbel 25.9–34.5% HL vs. 33.9–52.9; length of maxillary barbel 77.2–109.0% HL vs. 103.8–161.0).
Description:
Biometric data in Table 4.
Head depressed, body subcylindrical. Dorsal profile rising evenly from tip of snout to origin of dorsal fin, and then sloping gently ventrally from origin of dorsal fin to end of caudal peduncle. Ventral profile flat to anal-fin base, then sloping gently dorsally from anal-fin base to end of caudal peduncle. Anus and urogenital openings located at vertical through middle of adpressed pelvic fin. Skin granular, with prominent tubercles sparsely distributed on base of adipose and caudal fins. Lateral line complete and midlateral, laterosensory pores rimmed in beige. Anterior nuchal plate element triangular, with concave anterolateral margins and extensive contact with posterior nuchal plate element, but lacking saddle-shaped extensions. Vertebrae 16 + 21 = 37 (10) or 16 + 22 = 38 (6).
Head depressed and broad, triangular when viewed laterally. Snout convex when viewed from above. Anterior and posterior nares separated only by base of nasal barbel. Eyes small and ovoid, horizontal axis longest, located on dorsal half of head. Gill openings broad, extending from directly beneath post-temporal to isthmus.
Barbels in four pairs. Maxillary barbel thick, extending beyond base of last pectoral-fin ray. Nasal barbel slender, extending to anterior orbital margin. Inner mandibular barbel extending to middle of thoracic adhesive apparatus. Outer mandibular barbel originating posterolateral of inner mandibular barbel, extending to base of pectoral-fin spine.
Mouth inferior, premaxillary tooth band almost entirely exposed when mouth closed. Oral teeth small and villiform, in irregular rows on all tooth-bearing surfaces. Premaxillary teeth in single crescentic-shaped band. Dentary teeth in two patches separated by narrow gap at midline. Palate edentate.
Dorsal fin of subequal distance from snout tip to adipose-fin origin, with I,6 rays; dorsal margin truncate; anterior margin of spine smooth, posterior margin with weak serrations. Adipose fin straight anteriorly and angular posteriorly. Caudal fin deeply forked, lobes almost equal, with i,7,7,i (8) or i,7,8,i (16) principal rays. Procurrent rays symmetrical, extending only slightly anterior to fin base and consisting of five to eight rays. Anal-fin origin slightly anterior to vertical through adipose-fin origin. Anal fin with straight anterior margin and straight or slightly concave posterior margin; with ii,9 (7), ii,10 (16), or ii,11 (4) rays. Pectoral fin with I,8 (6), I,9 (20), or I,10 (1) rays; posterior fin margin straight; anterior spine margin smooth, posterior spine margin with nine to 12 serrations. Pelvic-fin origin at vertical through posterior end of dorsal-fin base. Pelvic fin with slightly convex margin and i,5 rays; tip of adpressed fin not reaching anal-fin origin.
Thoracic adhesive apparatus present, consisting of skin ridges (striae) in an elongate oval field extending from isthmus to end of pectoral-fin base and with median depression present on posterior one-third to half. Striae orientated anterodistally, radiating from median depression.
Coloration:
In 75% ethanol: dorsal surface of head and body brown, lateral surfaces of body ventrally becoming paler immediately below lateral line. Ventral surfaces of head and body greyish beige. Dorsal surface of head with distinct pale patches; nuchal plate elements distinctly pale. A distinct, thin, light grey mid-dorsal stripe outlining distal tips of neural spines extending from base of last dorsal-fin ray to adipose-fin origin. Laterosensory pores along lateral line rimmed in beige, imparting appearance of a narrow, pale line. Dorsal and anal fins, and dorsal surfaces of pectoral and pelvic fins with dark brown bases and bands on middle third, rest of fins yellow to hyaline. Adipose fin yellow at base and distally, remainder of fin dark brown. Caudal fin brown, with tips of lobes hyaline. Maxillary and nasal barbels brown dorsally, light yellow ventrally.
Distribution:
Known from the Fugong River, a tributary of the Xiaohei River, a tributary of the Nanting River, itself a tributary to the Salween River (Nujiang); and the Mengnong River, a tributary of the Nangun River, itself a tributary to the Salween River (Fig. 7).
Etymology:
The specific name fucatus is from the Latin meaning painted or coloured, in reference to the unusual colour pattern of the lateral surfaces of this species.
DISCUSSION
Wu et al. (1981) described G. zanaensis from Zana (28°29'28.7''N, 98°27'22.7''E) in the Nujiang (upper Salween River) drainage in Tibet. This species was not mentioned in Li's (1984) review of the Chinese Glyptothorax, in which G. longinema from Liuku Township in the Nujiang drainage and G. rubermentus from Wayao Township in the Lancangjiang drainage were described. Mo & Chu (1986) subsequently considered these two nominal species as synonyms of G. zanaensis. Subsequent studies have followed the result of Mo & Chu (1986), in considering G. zanaensis to be a species widely distributed from the Nujiang eastwards to the Lancangjiang drainages (Thomson & Page, 2006; Ferraris, 2007). Our study restricts the distribution of G. zanaensis to the upper Salween River (Nujiang) in Tibet and Yunnan, and revalidates G. longinema for populations from the upper Salween (Nujiang) and upper Mekong (Lancanjiang) drainages in Yunnan previously identified as G. zanaensis. Although our phylogenetic analyses recovered the Salween and Mekong River clades as reciprocally monophyletic, we were unable to distinguish between these two populations using morphology alone (Table 4). Therefore, we have considered these two populations to be conspecific. Glyptothorax zanaensis has once been recorded from the middle Mekong in Laos (Kottelat, 2001), but a preliminary study by H-H. Ng has revealed that this material is referable to a distinct, unnamed species (Ng & Rainboth, 2008).
Liu et al. (2010) studied the genetic diversity of G. zanaensis based on six populations in the main stream of the Nujiang drainage and Yuan et al. (2011) conducted a similar study on seven populations found in tributaries of the Nujiang. However, the latter study did not include the results of the former in its analysis. Both studies revealed significant haplotype diversity in the studied populations, especially in populations from Nujiang Prefecture (from Gongshan to Lushui County), implying the occurrence of incipient speciation. However, because these studies did not include morphological data for comparison, we are unable to place the results of these studies in the context of ours, although we hypothesize that more than one species may have been involved in the studies of Liu et al. (2010) and Yuan et al. (2011).
We have identified possible ontogenetic change in the tuberculation of G. granosus that may serve as a possible diagnostic character for this species. Twelve juvenile specimens of G. granosus(40.5–57.8 mm SL) that we examined possess prominent tubercles arranged in longitudinal rows along the flanks (Fig. 11), similar to the condition seen in species of the akysid catfish Pseudobagarius. Given that this skin morphology is unusual for Glyptothorax and has yet to be seen in many other congeners examined (other congeners typically lack such a marked dimorphism in tubercle morphology, with the juveniles showing little or no differences in the pattern of skin tuberculation from adults), the possibility is strong that this could be a useful character that diagnoses G. granosus.
Glyptothorax granosus, CAS 222110, paratype, 47.0 mm SL, showing the unusual tuberculation pattern in juveniles of this species.
Seven Glyptothorax species are known from the middle and lower parts of the Irrawaddy, Mekong, and Salween River drainages: G. dorsalis(Salween), Glyptothorax filicatus(Mekong), Glyptothorax horai(Mekong), G. lampris(Mekong), G. laosensis(Mekong), Glyptothorax panda(Irrawaddy), and Glyptothorax rugimentum(Salween). An additional four species are also known from the Upper Irrawaddy River drainage (in the Chindwin subdrainage) in north-eastern India: Glyptothorax chindwinica, Glyptothorax granulus, Glyptothorax minutus, and Glyptothorax ventrolineatus. These 11 species will be briefly compared to those treated in our study above below. Glyptothorax chindwinica is distinguished from G. fucatus, G. granosus, G. longinema, and G. zanaensis in having the ridges of the thoracic adhesive apparatus enclosing an almost circular depressed region in the centre (vs. depressed area in the thoracic adhesive apparatus not wholly enclosed by ridges) and a longer head (28.3–29.0% SL vs. 21.7–27.7). Glyptothorax dorsalis differs from G. fucatus, G. granosus, G. longinema, and G. zanaensis in having a longer dorsal-fin spine (16.3–22.2% SL vs. 10.3–16.8) and the presence (vs. absence) of dark spots on the body. Glyptothorax filicatus is distinguished from G. fucatus, G. granosus, G. longinema, and G. zanaensis in having a longer head (27.6–29.3% SL vs. 21.7–27.7) and in having (vs. lacking) dark spots on the body. Glyptothorax granulus differs from G. fucatus, G. granosus, G. longinema, and G. zanaensis in having a broad v-shaped depression in the posterior region of the thoracic adhesive apparatus (vs. depression small and almost indistinguishable or in a very narrow v-shape). Glyptothorax horai is distinguished from G. fucatus, G. granosus, G. longinema, and G. zanaensis in having (vs. lacking) dark spots on the body. Glyptothorax lampris differs from G. fucatus, G. granosus, G. longinema, and G. zanaensis in having conspicuous light patches on a predominantly dark body (vs. patches absent). Both G. laosensis and G. ventrolineatus are distinguished from G. fucatus, G. granosus, G. longinema, and G. zanaensis in having a dark body with distinct, pale, longitudinal stripes running along the lateral line and the mid-dorsal region of the body posterior to the dorsal fin (vs. such a colour pattern absent). Glyptothorax minutus differs from G. fucatus, G. granosus, G. longinema, and G. zanaensis in reaching a much smaller size (to 36 mm TL vs. to at least 96 mm SL) and in having a colour pattern consisting of dark saddles overlying a light body (vs. saddles absent). Glyptothorax panda is distinguished from G. fucatus, G. granosus, G. longinema, and G. zanaensis in reaching a much smaller size (to 32 mm SL vs. to at least 96 mm SL) and in having a more slender body (10.4–11.8% SL vs. 11.4–21.1) and a colour pattern consisting of dark saddles overlying a light body (vs. saddles absent). Glyptothorax rugimentum differs from G. fucatus, G. granosus, G. longinema, and G. zanaensis in having (vs. lacking) the ridges of the thoracic adhesive apparatus extending onto the gular region and a shorter adipose-fin base (9.4–13.5% SL vs. 12.8–19.6).
Most recent studies on the evolution of Chinese sisorids have focused on glyptosternines (Guo, He & Zhang, 2005; Peng et al., 2006). Given that glyptosternine catfishes are highly rheophilic and are solely associated with highly elevated fluviatile systems, it is therefore to be expected that a close correlation would exist between their diversification and the uplift of the Qinghai−Tibet Plateau. The same may not be true of Glyptothorax, because it is not restricted to high-elevation river systems. In this case, we would expect other vicariant events such as drainage captures to play a more important role in speciation, as has been demonstrated for other fish species occurring in lowland rivers (e.g. Rüber et al., 2004).
We recovered rapid speciation within the clade comprising G. zanaensis and closely related species during the middle Pliocene to early Pleistocene (2.68–1.21 Mya; C5–C11 in Fig. 3). This is congruent with the first phase of the uplifting of the Qinghai−Xizang Plateau, commonly referred to as the ‘Qingzang (Tibet) Movement’ (Li et al., 1996) and which took place 3.6–1.7 Mya. Although it has been generally accepted that significant changes in the palaeodrainage patterns took place before or during the uplift of the Qinghai−Xizang Plateau (Clark et al., 2004), the nature and timing of these changes are still open to debate. Conventional reconstructions of the palaeodrainage patterns suggest that the upper Salween (Nujiang) and upper Mekong (Lancangjiang) previously formed the headwaters of the palaeo-Red River, and that progressive drainage capture led to the current drainage patterns (Clark et al., 2004). However, the timing of this capture has been subject to some debate, with estimates ranging from 10 Mya (Clark et al., 2004) to before 24 Mya (Clift, Blusztajn & Nguyen, 2006). There is also evidence to suggest that the Nujiang and Lancangjiang were never connected to the Red River (Clift et al., 2008). Evidence from our data thus appears to indicate that vicariance from drainage captures may play a very little role (if any at all) in the speciation within some subclades of Glyptothorax, but further work is needed to fully verify this.
COMPARATIVE MATERIAL
Glyptothorax burmanicus:
KIZ 2002002385 (1), 99.5 mm SL; KIZ 2002002388 (1), 101.1 mm SL; KIZ 2002002393–2002002394 (2), 100.6–111.5 mm SL; KIZ 2002002397 (1), 107.5 mm SL; China: Yunnan, Yongde County, Daxueshan, Nanting River (a tributary of Nujiang). KIZ 2002001886–2002001887 (2), 116.4–142.5 mm SL; China: Yunnan, Yongde County, Daxueshan, Baishitou River. KIZ 2000000505–2000000507 (3), 219.6–265.6 mm SL; China: Yunnan, Lushui County, Liuku. KIZ 1983000726–1983000727 (2), 78.5–92.4 mm SL; KIZ 1983000729 (1), 72.2 mm SL; China: Yunnan, Changning County, and Yun County. KIZ 1976000841–1976000843 (3), 144.6–164.7 mm SL; KIZ 2001003749 (1), 195.2 mm SL; China: Yunnan, Tengchong County. KIZ 2003004654–2003004655 (2), 160.3–194.2 mm SL; China: Yunnan, Longling County, Tengchongqiao. KIZ 1998000616 (1), 194.6 mm SL; China: Yunnan, Tengchong County, Longjiangqiao. KIZ 1983000689 (1), 72.8 mm SL; KIZ 1983000691 (1), 67.8 mm SL; KIZ 1983000702 (1), 79.8 mm SL; KIZ 1983000706 (1), 69.3 mm SL; China: Yunnan, Yingjiang County, Zhefang. BMNH 1987.9.17.4 (1), 157.4 mm SL, China: Yunnan, Tengchong County, Tuantian. NRM 39941 (2), 60.8–72.8 mm SL, Myanmar: Kachin State, Myitkyina market. ZRC 46126 (1), 247.3 mm SL, China: Yunnan, Baoshan, Nujiang at old road from Baoshan to Tengchong (purchased in Tengchong). UMMZ 246860 (6 alc., 1 c&s), 40.4–83.9 mm SL, Thailand: Mae Hong Son, Huai Mae Saloh, tributary of Salween River north of Mae Sam Laep. ZRC 50595 (12), 93.4–115.5 mm SL, China: Yunnan, Linchang, Nanding River at Xingfu village, 47 km on road from Linchang to Yunxian, 24°10'27.0''N, 100°3'18.6''E.
1. Thoracic adhesive apparatus extending onto gular region 2
–Thoracic adhesive apparatus not extending onto gular region 3
2. Flanks with irregular dark blotches; head and body not prominently tuberculate; pectoral spine with seven to eight serrations; dorsal-fin base 11.0–13.2% SL; pectoral-fin length 15.6–19.6% SL; caudal peduncle depth 8.6–9.8% SL G. obliquimaculatus
–Flanks without prominent dark blotches; head and body prominently tuberculate; pectoral spine with ten to 12 serrations; dorsal-fin base 13.7–15.8% SL; pectoral-fin length 20.2–22.6% SL; caudal peduncle depth 6.1–7.6% SL G. rugimentum
3. Tooth band on upper jaw very broad, forming roof of oral cavity; thoracic adhesive apparatus with ovoid central pit G. burmanicus
–Tooth band on upper jaw narrow, restricted on anterior margin of oral cavity; thoracic adhesive apparatus without ovoid central pit 4
4. Prominent pale longitudinal stripe along lateral line, broader than lateral line G. trilineatus
–Prominent pale longitudinal stripe along lateral line absent, or if present, as wide as or narrower than lateral line 5
5. Flanks pale or predominantly pale, with scattered dark spots frequently present 6
–Flanks dark, without dark spots 7
6. Nuchal plate elements frequently pale and strongly contrasting with rest of predorsal region; prominent pale patches on flanks; caudal peduncle depth 6.9–7.9% SL G. dorsalis
–Nuchal plate elements frequently dark and not strongly contrasting with rest of predorsal region; flanks uniform or nearly so, without prominent pale patches; caudal peduncle depth 5.3–6.9% SL G. ngapang
7. Flanks dark, fading to paler colour ventrally; triangular anterior nuchal plate element with concave anterolateral edges and extensive contact with posterior nuchal plate element, lacking saddle-shaped lateral extensions G. fucatus sp. nov.
–Flanks uniformly dark; triangular anterior nuchal plate element either with straight anterolateral edges and lacking extensive contact with posterior nuchal plate element, or with concave anterolateral edges, extensive contact with posterior nuchal plate element and saddle-shaped lateral extensions 8
8. Diverging pattern of striae running along edges of median depression in thoracic adhesive apparatus G. zanaensis
–Striae radiating in single series along median depression in thoracic adhesive apparatus 9
9. Anterior nuchal plate elements with lateral saddle-shaped extensions; depth of caudal peduncle 5.7–7.6% SL; body depth at anus 12.9–16.5% SL; head width 16.6–19.5% SL G. granosus sp. nov.
–Anterior nuchal plate elements without lateral saddle-shaped extensions; depth of caudal peduncle 7.1–10.6% SL; body depth at anus 14.6–20.4%% SL; head width 19.3–22.9% SL G. longinema
Glyptothorax chindwinica:
Data from Vishwanath & Linthoingambi (2007).
Glyptothorax deqinensis:
KIZ 1974000937–1974000938 (two paratypes), 89.2–89.7 mm SL; 941 (one paratype), 94.8 mm SL; China: Yunnan, Deqin County, Yanmen. KIZ 1980001799 (one paratype), 85.1 mm SL; China: Yunnan, Zidongjiang. KIZ 2003007931 (1), 84.4 mm SL; China: Yunnan, Deqin County, Yunling. KIZ 20040929229 (1), 95.3 mm SL; 233 (1), 80.9 mm SL; China: Yunnan, Baoshan, Wayao Township, Fanrong village. KIZ 2005000495 (1), 79.8 mm SL; China: Yunnan, Weixi County, Badi Township. BMNH 1987.9.17.5 (one paratype), 74.0 mm SL; China: Yunnan, Weixi, Baiji.
Glyptothorax dorsalis:
CMK 17785 (9), 40.0–65.5 mm SL, Myanmar: Kayin, Ataran, stream ‘Chon Son’ between Kyondaw and Phadaw, about 20 km north-west of Payathouzou (at border with Thailand), 15°25'N, 98°15'E.
Glyptothorax filicatus:
ZFMK 34398 (holotype), 83.3 mm SL; UMMZ 245668 (one paratype), 69.0 mm SL; ZFMK 34399 (one paratype), 91.9 mm SL Vietnam: Thua Thien Hue province, stream draining to Se Sap River, 12 km east of A Luoi, 16°20.57'N, 107°9.41'E.
Glyptothorax granulus:
Data from Vishwanath & Linthoingambi (2007).
Glyptothorax horai:
UMMZ 240735 (10), 52.7–80.2 mm SL; Laos: Oudom Xai Province, Muang Xai market, Ko (Mekong) River drainage, 20°42'N, 101°58'E. UMMZ 241061 (2), 81.5–111.5 mm SL; Laos: Luang Prabang Province, Nam Xuang, 4 km downstream from Ban Pak Kung, Mekong River drainage, 20°3'N, 102°22'E. Additional data from Fowler (1934).
Glyptothorax lampris:
KIZ 1996003025–1996003027 (3), 45.6–61.9 mm SL; KIZ 1973001514 (1), 47.5 mm SL; KIZ 1973001517 (1), 48.2 mm SL; KIZ 1973001358–1973001359 (2), 50.3–99 mm SL; China: Yunnan, Mengla County, Menghan. KIZ 1996003028 (1), 59.1 mm SL; China: Yunnan, Mengla County, Menglun. KIZ JWS20100023 (1), 47.4 mm SL; China: Yunnan, Lancang County, Nuozhaidu. ANSP 59357 (holotype), 41.8 mm SL; Thailand: Chieng Mai. UMMZ 232349 (1), 50.4 mm SL; Cambodia: Stung Treng, Tonle San rapids, 9 km upstream from confluence with Tonle Kong, 13°34'N, 106°7'E. UMMZ 234522 (4), 38.1–47.7 mm SL; Cambodia: Kompong Speu Province, Prek Thnot at Kompong Speu. UMMZ 240662 (9), 37.0–57.0 mm SL; Laos: Luang Prabang Province, Nam Xuang at Ban Pak Xuang. UMMZ 245600 (2), 66.2–67.88 mm SL; Thailand: Surat Thani Province, Klong Sok at Phanom.
Glyptothorax laosensis:
KIZ 2009000498–2009000500 (3), 70.1–82.1 mm SL; KIZ 2009000502 (1), 82.2 mm SL; KIZ 2009000504 (1), 62.7 mm SL; KIZ 2009000506 (1), 51.0 mm SL; China: Yunnan, Yun County, Dachaoshan. KIZ 2007003991, KIZ 2007003999–2007004000 (3), 63.3–67.4 mm SL; China: Yunnan, Mengla County, Menglun. KIZ 2009002360 (1), 106.9 mm SL; China: Yunnan, Jinggu County, Weiyuanjiang. KIZ 2009001173 (1), 73.2 mm SL; KIZ 2009001177–2009001178 (2), 53.9–65.9 mm SL; China: Yunnan, Lancang County, Nuozhaidu. KIZ 2005012191–2005012193 (3), 74.5–81.4 mm SL; KIZ 2005012195 (1), 81.1 mm SL; China: Yunnan, Gengma County, Hepai, Dangpa River. KIZ 1986003690 (1), 58.8 mm SL; KIZ 1986003692–1986003693 (2), 59.3–76.6 mm SL; China: Yunnan, Jinghong. ANSP 59412, holotype, 51.3 mm SL; Thailand: Bua Yai. ZFMK 21724–21755 (32), 33.9–53.5 mm SL; Vietnam: Lam Dong province, Dai Tan River about 30 km south of Da Lat, 11°46'38.4''N, 108°19'16.8''E. ZFMK 24195 (1), 76.0 mm SL; Vietnam: Dac Lac province, small stream at Ea Nuol about 20 km north-west of Buon Ma Thuot, 12°41'34.8''N, 107°57'44.4''E. ZFMK 24237 (1), 81.7 mm SL; Vietnam: Dac Lac province, Ea Tul stream at Ban Tuan, a tributary of the Srepok River. ZFMK 24321–24329 (9), 43.2–93.7 mm SL; Vietnam: Dac Lac province, stream about 20 km south of Buon Ma Thuot, a tributary of the Srepok River, 12°32'26.4''N, 107°52'30.6''E. ZFMK 25037–25039 (3), 35.0–67.7 mm SL; Vietnam: Dac Lac province, Crong Pach stream about 50 km east of Buon Ma Thuot, a tributary of the Srepok River, 12°46'18.6''N, 108°22'33.6''E.
Glyptothorax longicauda:
BMNH 1987.9.17.13 (one paratype), 149.0 mm SL; China: Yunnan, Tengchong County, Guyong. KIZ 2003006952–2003006956 (5), 76.6–210.1 mm SL; China, Yunnan, Yingjiang County, Kachang, Mengdian River. KIZ 05641 (1), 125.8 mm SL; KIZ 05594 (1), 154.2 mm SL; China: Yunnan, Tengchong County, Houqiao, Binglangjiang River. KIZ 05526 (1) 103.4 mm SL; China: Yunnan, Tengchong County, Xixiaohe, Reshuitang. KIZ 05346 (1), 117.8 mm SL; China: Yunnan, Tengchong County, Beihaihu, Longchuanjiang. KIZ 05514 (1), 138.5 mm SL; KIZ 05698 (1), 123.5 mm SL; KIZ 05737 (1), 120.9 mm SL; China: Yunnan, Tengchong County, near Lianmengjie, Longchuanjiang. KIZ 1978001648 (1), 179.1 mm SL; KIZ 1978000888 (1), 153.4 mm SL; China: Yunnan, Tengchong County, Guyong Township, Xima, Tengbiguan. KIZ 1976001249–1976001250 (2), 145.1–185.9 mm SL; China: Yunnan, Tengchong County, Qushi. CAS 226065 (1), 112.9 mm SL; ZRC 52360 (2), 80.7–99.7 mm SL; China: Yunnan, Binglangjiang at Houqiao village, 25°21'N, 98°12'E. ZRC 51497 (3), 67.7–151.4 mm SL; China: Yunnan, Dayingjiang drainage, Liao River.
Glyptothorax longjiangensis:
KIZ 20000004228 (1), 100.6 mm SL; 230 (1), 97.0 mm SL; 234 (1), 81.2 mm SL; KIZ 07153 (1), 115.5 mm SL; China: Yunnan, Longling County, Tenglongqiao. KIZ 2006001775–2006001777 (3), 84.7–119.7 mm SL; China: Yunnan, Tengchong County, Tenglongqiao. KIZ 2006010174–2006010178 (5), 94.8–122.9 mm SL; China: Yunnan, Tengchong County, Wuhe, Longchuanjiang. KIZ 2006009825 (1), 95.7 mm SL; KIZ 2006009827–2006009828 (2), 89.6–100.9 mm SL; KIZ 2006009869–2006009871 (3), 99–123.5 mm SL; KIZ 05722 (1), 96.1 mm SL; KIZ 05736 (1), 107.9 mm SL; KIZ 05741 (1), 87.9 mm SL; China: Yunnan, Tengchong County, near Lianmengjie, Longchuanjiang. KIZ 07143–07144 (2), 87.3–96.3 mm SL; China: Yunnan, Tengchong County, Tuantian.
Glyptothorax macromaculatus:
KIZ 1974001228 (holotype), 80.1 mm SL; KIZ 1998004846–1998004847 (2), 69.1–71 mm SL; KIZ 1998004850–1998004851 (2), 78.8–81.8 mm SL; China: Yunnan, Yangbi County. KIZ 2005012201 (1), 112.3 mm SL; KIZ 2005012203–2005012204 (3), 71–90.7 mm SL; China: Yunnan, Gengma County, Hepai, Dangpa River. KIZ 2009001692–2009001693 (2), 69.2–86.8 mm SL; KIZ 2009001699 (1), 78.3 mm SL; China: Yunnan, Jinggu County, Zhengxing, no. 1 Bridge. KIZ 2008000107–2008000108 (2), 70.5–70.6 mm SL; KIZ 2008000111 (1), 57.4 mm SL; China: Yunnan, Lancang County, Menggeng. KIZ 2007003950–2007003952 (3), 77.8–111.6 mm SL; China: Yunnan, Mengla County, Menglun. CMK 5612 (5), 55.8–76.1 mm SL; China: Yunnan, Yangbi River from its confluence with Erhai River to about 20 km upstream of Yangbi.
Glyptothorax minimaculatus:
BMNH 1987.9.17.16 (one paratype), 132.0 mm SL; China: Yunnan, Tengchong County, Qushi. KIZ 05482 (1), 68.2 mm SL; KIZ 05484–05485 (2), 70.0–73.9 mm SL; China: Yunnan, Tengchong County, Dayingjiang. KIZ 8043218 (1), 94.3 mm SL; KIZ 8043220 (1), 81.9 mm SL; China: Yunnan, Tengchong County, Qushi.
Glyptothorax minutus:
Data from Hora (1921).
Glyptothorax ngapang:
KIZ 2003004905–200300407 (3), 84.8–107.8 mm SL; KIZ 07265–07266 (2), 103.5–103.9 mm SL; China: Yunnan, Longling County, Gongyang River (a tributary of Nujiang). KIZ 2002002324–2002002325 (2), 79.2–79.8 mm SL; KIZ 2002002327 (1), 74.0 mm SL; China: Yunnan, Yongde County, Daxueshan, Nanting River (a tributary of Nujiang). KIZ 2002001767–69 (3), 80.8–102.1 mm SL; KIZ 2002001813 (1), 80.5 mm SL; KIZ 2002001817 (1), 102.4 mm SL; China: Yunnan, Yongde County, Daxueshan, Baishitou River. KIZ 2005012246–2005012248 (3), 63.4–107.8 mm SL; China: Yunnan, Gengma County, Mengding, Xiaohei River, a tributary of Nanting River. KIZ 1983000673–1983000674 (2), 67.1–104.6 mm SL; KIZ 1983001635 (1), 134.9 mm SL; China: Yunnan, Changning County. KIZ 1995000256–1995000257 (2), 84–94.6 mm SL; China: Yunnan, Cangyuan County, Nangun River. CMK 12173 (1), 60.0 mm SL; CMK 12174 (2), 45.1–49.5 mm SL, Thailand: Tak province, stream at km 57 on road from Mae Sot to Wa Rei (5 km before Wa Rei). CMK 18798 (3), 70.6–95.1 mm SL, Myanmar: Kayin, Ataran, stream ‘Chon Son’ between Kyondaw and Phadaw, about 20 km north-west of Payathouzou (at border with Thailand). NRM 32651 (2), 88.1–91.2 mm SL, China: Yunnan, Zhenkang, Nanbang River, tributary to Nanding River, about 5 km from Mengdui on the road to Zhenkang. NRM 40698 (7), 105.3–135.8 mm SL, Myanmar: Kachin, Myitkyina market.
Glyptothorax obliquimaculatus:
KIZ 200504151904 (holotype), 85.4 mm SL; KIZ 200504151901–20050415903 (three paratypes), 71.6–74.6 mm SL; KIZ 20050415905 (one paratype), 73.3 mm SL; KIZ 20050415907–20050415914 (eight paratypes), 66.7–80.1 mm SL; China, Yunnan, Gengma County, Mengding, Xiaohei River (a primary tributary of the Nanting River which is itself a tributary to the Nujiang).
Glyptothorax panda:
UMMZ 246004 (8 alc., 1 c&s), 24.7–28.4 mm SL; Myanmar: Bago Division, Pyu township, Pyu stream (tributary of Sittang River) c. 229 km from Yangon, 18°29'N, 96°26'E. Additional data from Ferraris & Britz (2005).
Glyptothorax rugimentum:
ZRC 50572 (holotype), 78.6 mm SL; CMK 17784 (five paratypes), 64.5–75.6 mm SL; CMK 17950 (one paratype), 70.0 mm SL; Myanmar: Kayin State, Ataran drainage, stream ‘Chon Son’ between Kyondaw and Phadaw, about 20 km north-west of Payathouzu (at border with Thailand), 15°25'N, 98°15'E. CMK 16243 (three paratypes), 55.5–58.5 mm SL, Thailand: Mae Hong Son, Nam Mae Sariang at dam south-west of Amphoe Mae Sariang. UMMZ 245971 (two paratypes), 44.5–49.7 mm SL, Myanmar: Bago, Pyu township, Pyu stream (tributary of Sittang River) c. 229 km from Yangon, 18°29'N, 96°26'E. UMMZ 246033 (1 alc., 1 c&s paratypes), 60.5–68.9 mm SL, Thailand: Salween River drainage. UMMZ 247301 (12 paratypes), 43.0–53.7 mm SL, Myanmar: Ataran River drainage.
Glyptothorax trilineatus:
ZSI F10380/1 (two syntypes), 58.1–77.9 mm SL; Myanmar: Tenasserim. KIZ 2002002343 (1), 83.7 mm SL; KIZ 2002002345–2002002346 (2), 60.8–65.3 mm SL; China: Yunnan, Yongde County, Daxuesha, Nanting River (a tributary of Nujiang). KIZ 2002002508–2002002511 (4), 73.2–111.2 mm SL; China: Yunnan, Yongde County, Daxueshan, Nanjing River. KIZ 2005012228–2005012229 (2), 53.0–54.0 mm SL; China: Yunnan, Gengma County, Mengding, Xiaohei River, a tributary of Nanting River. KIZ 2003005047 (1), 49.0 mm SL; KIZ 2003005049 (1) 38.0 mm SL; KIZ 2006009862 (1), 50.6 mm SL; China: Yunnan, Longling County, Mengzhai River. KIZ 07263–07264 (2), 52.7–55.8 mm SL; KIZ 07285 (1) 59.3 mm SL; China: Yunnan, Longling County, Gongyang River. KIZ 2006009864 (1), 60 mm SL; China: Yunnan, Longling County, Taozhai River. KIZ 2006004164–2006004168 (5), 43.7–49.7 mm SL; KIZ 2006004170 (1), 45.4 mm SL; China: Yunnan, Longchuan County, Wangzishu. KIZ 2006003829 (1), 49.0 mm SL; China: Yunnan, Longchuan County, Mengyue. KIZ 1983000680 (1), 41.2 mm SL; China: Yunnan, Yingjiang County. CAS 210296 (1), 78.3 mm SL; Myanmar: Sagaing division, Alaungdaw Kathapa National Park, Paya Chaung, 22°18'49.6''N, 94°28'52.3''E. CAS 223041 (4), 35.8–44.7 mm SL; China: Yunnan, Baoshan Prefecture, Longling County, Menzhai River from roughly 100 m downstream of bridge to a point approx. 800 m upstream from bridge, 24°14'21.5''N, 99°1'1.1''E–24°14'29.1''N, 99°0'38.0''E.
Glyptothorax ventrolineatus:
Data from Vishwanath & Linthoingambi (2006).
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (31071907/30730017), Yunnan Provincial Science and Technology Program (2009CC008), Gaoligong Shan Biological Survey (DEB-0103795), and National Basic Research Program of China (2007CB411600). We are grateful to the following for allowing access to material under their care: James Maclaine (BMNH), David Catania (CAS), Maurice Kottelat (CMK), E. Zhang (IHB), Lina Du (KIZ), Sven Kullander (NRM), Douglas Nelson (UMMZ), Klaus Busse (ZFMK), Kelvin Lim (ZRC), and A. K. Karmakar (ZSI). We thank Zhisheng Wang (Nangunhe Nature Reserve), and Xiaofu Pan and Guohua Yu (both KIZ) for help in collecting specimens. We are also grateful to Li Jia and Jian Yang (both KIZ) for help during molecular experiments, and Weiying Wang, Guohua Yu, and Feng Dong (all KIZ) for assistance in data analysis.
REFERENCES
Author notes
Joint first authors: the authors wish it to be known that the first two authors contributed equally to this work.