Abstract
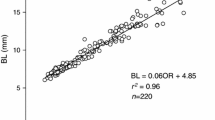
The ontogenetic changes in the growth potential of larval and juvenile laboratoryreared Pacific bluefin tuna were examined based on RNA-DNA and protein-DNA ratios. Experimental fish were reared at the Ohshima Experiment Station of Kinki University Fisheries Laboratory in August 2002. Samples were taken from 13 to 35 days after hatching (DAH). Metamorphosis from larva to the juvenile stage was observed around 23 DAH. Somatic growth of Pacific bluefin tuna was accelerated after metamorphosis. The value of the RNA-DNA ratio from 13 to 19 DAH increased slightly from 3.77±0.58 (mean±SD) to 7.28±2.23. After that, the ratio markedly increased from 13.89±3.71 on 21 DAH to 19.11±4.27 on 23 DAH, which was the end of the metamorphic period. After 25 DAH, the ratio remained at a high level of 15–20. The protein-DNA ratio showed a similar tendency to the RNA-DNA ratio. These results suggest that the rapid increase in the RNA-DNA ratio in the metamorphic period supports the consequent rapid somatic growth in the juvenile stage. The high ratio after the metamorphic period could be because of the species-specific traits large prey exhibit for their survival and because of the tuna’s fast-growth after the juvenile stage.
Similar content being viewed by others
References
Sawada Y, Okada T, Miyashita S, Murata O, Kumai H. Completion of the Pacific bluefin tuna Thunnus orientalis (Temminck et Schlegel) life cycle. Aquac. Res. 2005; 36: 413–421.
Kaji T, Tanaka M, Takahashi Y, Oka M, Ishibashi N. Preliminary observations on development of Pacific bluefin tuna Thunnus thynnus (Scombridae) larvae reared in the laboratory, with special reference to the digestive system. Mar. Freshwater Res. 1996; 47: 261–269.
Miyashita S, Sawada Y, Okada T, Murata O, Kumai H. Morphological development and growth of laboratory-reared larval and juvenile Thunnus thynnus (Pisces: Scombridae). Fish. Bull. (Wash. D. C.) 2001; 99: 601–616.
Takii K, Miyshita S, Seoka M, Tanaka Y, Kubo Y, Kumai H. Changes in chemical contents and enzyme activities during embryonic development of bluefin tuna. Fish. Sci. 1997; 63: 1014–1018.
Miyashita S, Kato K, Sawada Y, Murata O, Ishitani Y, Shimizu K, Yamamoto S, Kumai H. Development of digestive system and digestive enzyme activity of larval and juvenile Bluefin tuna, Thunnus thynnus, reared in the laboratory. Aquac. Sci. 1998; 46: 111–120.
Sawada Y, Miyashita S, Aoyama M, Kurata M, Mukai Y, Okada T, Muarata O, Kumai H. Rotifer size selectivity and optimal feeding density of bluefin tuna, Thunnus thynnus, larvae. Aquac. Sci. 2002; 48: 169–177.
Hunter JR. Feeding ecology and predation of marine fish larvae. In: Lasker R (ed.). Marine Fish Larvae, University of Washington Press, Seattle. 1981; 33–77.
Tanaka Y, Satoh K, Iwahashi M, Yamada H. Growth dependent recruitment of Pacific bluefin tuna Thunnus orientalis in the northwestern Pacific Ocean. Mar. Ecol. Prog. Ser. 2006; 319: 225–235.
Buckly LJ. Changes in ribonucleic and deoxyribonucleic acid and protein 10 content during ontogenesis in winter flounder Pseudopleuronectes americanus and effect of starvation. Fish. Bull. (Wash. D.C.) 1980; 77: 703–708.
Buckly LJ. RNA-DNA ratio: an index of larval fish growth in the sea. Mar. Biol. 1984; 80: 291–298.
Ferron A, Leggett WC. An appraisal of condition for marine fish larvae. Adv. Mar. Biol. 1994; 30: 217–303.
Bergeron JP. Nucleic acids in ichthyoplankton ecology: a review, with emphasis on recent advances for new perspectives. J. Fish Biol. 1997; 51: 284–302.
Buckly LJ, Calderone E, Ong TL. RNA-DNA ratio and other nucleic acid-base indicators for growth and condition of marine fishes. Hydrobiology 1999; 401: 265–277.
Gwak WS, Tanaka M. Developmental change in RNA:DNA ratios of fed and starved laboratory-reared Japanese flounder larvae and juveniles, and its application to assessment of nutritional condition for wild fish. J. Fish Biol. 2001; 59: 902–915.
Bullow FJ. RNA-DNA ratios as an indicator of recent growth rates of a fish. J. Fish. Res. Board Can. 1970; 27: 2343–2349.
Hovenkamp F, Witte JJ. Growth, otolith growth and RNA:DNA ratios of larval plaice Pleuronectes platessa in the North Sea 1987–1989. Mar. Ecol. Prog. Ser. 1991; 70: 105–116.
Westerman M, Holt GJ. RNA:DNA ratio during the critical period and early larval growth of red drum Sciaenops ocellatus. Mar. Biol. 1994; 121: 1–9.
Clemessen CM. Improvements in fluorometric determination of the RNA and DNA content of individual marine fish larvae. Mar. Ecol. Prog. Ser. 1993; 100: 177–183.
Sato C, Kimura R, Nakata K, Umeda S, Suzuki M. RNA/DNA ratio of first-feeding larvae of Japanese sardine. Fish. Sci. 1995; 61: 538–539.
Kaji T, Tanaka M, Oka M, Takeuchi H, Ohsumi S, Teruya K, Hirokawa J. Growth and morphological development of laboratory-reared yellowfin tuna Thunnus albacares larvae and juveniles, with special emphasis on the digestive system. Fish. Sci. 1999; 65: 700–707.
Young JW, Davis TLO. Feeding ecology of larvae of southern bluefin, albacore and skipjack tunas (Pisces: Scombridae) in the eastern Indian Ocean. Mar. Ecol. Prog. Ser. 1990; 61: 17–29.
Gwak WS, Tanaka M. Changes in RNA, DNA and protein contents of laboratory-reared Japanese flounder Paralichthys olivaceus during metamorphosis and settlement. Fish. Sci. 2002; 68: 27–33.
Clemmesen CM. Importance and limits of RNA/DNA ratios as a measure of nutritional condition in fish larvae. In: Watanabe Y, Yamashita Y, Oozeki Y (eds). Survival strategies in Early Life Stages of Marine Resources. A. A. Balkema, Rotterdam. 1996; 141–151.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, Y., Gwak, WS., Tanaka, M. et al. Ontogenetic changes in RNA, DNA and protein contents of laboratory-reared Pacific bluefin tuna Thunnus orientalis . Fish Sci 73, 378–384 (2007). https://doi.org/10.1111/j.1444-2906.2007.01345.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1444-2906.2007.01345.x