Abstract
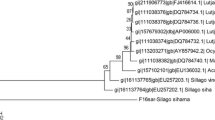
A total of 110 adult individuals from four ommastrephid (family Ommastrephidae) squid species (Ommastrephes bartramii, Sthenoteuthis oualaniensis, Eucleoteuthis luminosa, and Hyaloteuthis pelagica) were used to obtain diagnostic DNA markers for species identification. Restriction fragment length polymorphism (RFLP) analysis of a partial segment (855 bp) of the mitochondrial DNA cytochrome oxidase I (COI) gene amplified by polymerase chain reaction (PCR) revealed that the restriction profiles of two endonucleases (Alu I and Tsp509 I) were diagnostic for species identification. The restriction assay partially supplemented with nucleotide sequence analysis successfully assigned 69 damaged and morphologically equivocal ommastrephid paralarvae collected in northern Hawaiian waters, identifying 60 O. bartramii, eight S. oualaniensis, and one E. luminosa. The family Ommastrephidae appears to be monophyletic. Although the phylogenetic relationships among genera were not resolved well due to apparent homoplasy and large genetic divergence between species, COI sequence data without transitions provided support for subfamily level relationships.
Similar content being viewed by others
References
Okutani T, Cuttlefish and Squids of the world in Color. National Cooperative Association of Squid Processors, Tokyo, 1995.
Ichii T. The North Pacific Ocean sea area. In: Nasu K, Okutani T, Ogura M (eds). Squids — from the Organism to Consumption. 3rd edn. Seizando Shoten Publishing Co., Tokyo. 2002; 195–209 (in Japanese).
Harman RF, Young RE. The larvae of ommastrephid squids (Cephalopoda, Teuthoidea) from Hawaiian waters. Vie Milieu 1985; 35: 211–222.
Wormuth JH, O’Dor RK, Balch N, Dunning MC, Förch EC, Harman RF, Rowell TW. Family ommastrephidae steenstru. In: Sweeney M, Roper CFE, Mangold KM, Clarke MR, Boletzky Sv (eds). ‘Larval’ and Juvenile Cephalopods: A Manual for Their Identification. Smith. Contr. Zool. 1992; 513: 105–119.
Mori J, Tanaka H, Yatsu A. Paralarva and adults of neon flying squid (Ommastrephes bartramii) occurred in the subtropical North Pacific Ocean in autumn. Report of the 1997 Meeting on Squid Resources. Tohoku Nat. Fish. Res. Inst. Hachinohe, 1999; 1–8.
Mori J, Okazaki M, Tanaka H, Yatsu A. Spawning ground surveys of Ommastrephes bartramii in the Subtropical North Pacific Ocean in autumn, 1997 and 1998. Report on the 1998 Meeting of Squid Resources. Hokkaido Nat. Fish. Res. Inst. Kushiro. 1999; 85–86.
Dunning M. A review of the systematics, distribution, and biology of the Arrow squid genera Ommastrephes Orbigny, 1835, Sthenoteuthis Verrill, 1880, and Ornithoteuthis Okada, 1927 (Cephalopoda: Ommastrephidae). Smith. Contr. Zool. 1998; 586: 425–433.
Wormuth JH. A brief history of their systematics and a review of the systematics, distribution, and biology of the genera Martialia Rochebrune and Mabille, 1889, Todaropsis Girard, 1890, Dosidicus Steenstrup, 1857, Hyaloteuthis Gray, 1849, and Eucleoteuthis Berry, 1916. Smith. Contr. Zool. 1998; 586: 373–383.
Young RE, Hirota J. Description of Ommastrephes bartramii (Cephalopoda: Ommastrephidae) paralarvae with evidence for spawning in Hawaiian waters. Pac. Sci. 1990; 44: 71–80.
Wakabayashi T, Saito K, Tsuchiya K, Segawa S. Descriptions of Eucleoteuthis luminosa (Sasaki, 1915) and Ornithoteuthis volatilis (Sasaki, 1915) paralarvae in the northwestern Pacific. Venus 2002; 60: 237–260.
Young RE. Aspects of the natural history of pelagic cephalopods of the Hawaiian mesopelagic boundary region. Pac. Sci. 1995; 49: 143–155.
Bower JR, Seki MP, Young RE, Bigelow KA, Hirota J, Flament P. Cephalopod paralarvae assemblages in Hawaiian Island waters. Mar. Ecol. Prog. Ser. 1999; 185: 203–212.
Chow S, Clarke ME, Walsh PJ. PCR-RFLP analysis on thirteen western Atlantic snappers (subfamily Lutjaninae): a simple method for species and stock identification. Fish. Bull. 1993; 91: 619–627.
Smith PJ, Griggs L, Chow S. DNA identification of Pacific bluefin tuna (Thunnus orientalis) in the New Zealand fishery. N. Z. J. Mar. Freshwater Res. 2001; 35: 843–850.
Takeyama H, Chow S, Tsuzuki H, Matsunaga T. Mitochondrial DNA sequence variation within and between Thunnus species and its application to species identification. J. Fish Biol. 2001; 58: 1646–1657.
Anderson FE. Phylogeny and historical biogeography of the loliginid squids (Mollusca: Cephalopoda) based on mitochondrial DNA sequence data. Mol. Phylogenet., Evol. 2000; 15: 191–214.
Söller R, Warnke K, Saint-Paul U, Blohm D. Sequence divergence of mitochondrial DNA indicates cryptic biodiversity in Octopus vulgaris and supports the taxonomic distinctiveness of Octopus mimus (Cephalopoda: Octopodidae). Mar. Biol. 2000; 136: 29–35.
Kassahn KS, Donnellan SC, Fowler AJ, Hall KC, Adams M, Shaw P. Molecular and morphological analyses of the cuttlefish Sepia apama indicate a complex population structure. Mar. Biol. 2003; 143: 947–962.
Yokobori S, Fukuda N, Nakamura M, Aoyama T, Oshima T. Long-term conservation of six duplicated structural genes in cephalopod mitochondrial genomes. Mol. Biol. Evol. 2004; 21: 2034–2046.
Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: Molecular Evolutionary Genetics Analysis Software Arizona State University, Tempe. 2001.
Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods). Sinauer Associates. Sunderland. 2000.
Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics 1998; 14: 817–818.
Felsenstein J. PHYLIP (Phylogeny Inference Package), Version 3.6. Distributed by the Author. Department of Genome Sciences, University of Washington, Seattle. 2004.
Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003; 52: 696–704.
Saito H, Nakamura Y, Murata M. Distribution of cephalopod paralarvae in the northwest Pacific-morphological characters and distribution of ommastrephid paralarvae. Contr. Fish. Res. Jpn. Sea Block 1990; 17: 33–39. (in Japanese).
Carlini DB, Graves JE. Phylogenetic analysis of cytochrome c oxidase I sequences to determine higher-level relationships within the coleoid cephalopods. Bull. Mar. Sci. 1999; 64: 57–76.
Lindgren AR, Gribet G, Nishiguchi MK. A combined approach to the phylogeny of Cephalopoda (Mollusca). Cladistics 2004; 20: 454–486.
Roper CFE, Sweeny MJ, Nauen C. FAO species catalogue, 3. Cephalopods of the world. An annotated and illustrated catalogue of species of interest to fisheries. FAO Fisheries Synop. 1984; 125: 1–277.
Roeleveld MA. Genetic Interrelationships within the Ommastrephidae (Cephalopoda). In: Clark MR, Truemen ER (eds). The Mollusca, Vol. 12. Academic Press, New York. 1988; 277–291.
Yokawa K. Allozyme differentiation of sixteen species of ommastrephid squid (Mollusca, Cephalopoda). Antarctic Sci. 1994; 6: 201–204.
Lindgren AR, Katugin ON, Amezquita E, Nishiguchi MK Evolutionary relationships among squids of the family Gonatidae (Mollusca: Cephalopoda) inferred from three mitochondrial loci. Mol. Phylogenet. Evol. 2005; 36: 101–111.
Wormuth JH. The biogeography and numerical taxonomy of the oegopsid squid family Ommastrephidae in the Pacific Ocean. Bull. Scripps Inst. Ocean 1976; 23: 1–90.
Sasaki M. On three interesting new oegopsids from the Bay of Sagami. J. Coll. Agric. Tohoku Imp. Uni. 1915; 6: 131–150.
Wada S, Ichii T, Sakai M. Sequence comparison of 16SrRNA among six Ommastrephids. Report on the 2000 Meeting of Squid Resources. Nat. Res. Inst. Far Seas Fish. Shizuoka. 2002; 78–79.
Piertney SB, Hudelot C, Hochberg FG, Collins MA. Phylogenetic relationships among cirrate octopods (Mollusca: Cephalopoda) resolved using mitochondrial 16S ribosomal DNA sequences. Mol. Phyogenet Evol. 2003; 27: 348–353.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wakabayashi, T., Suzuki, N., Sakai, M. et al. Identification of ommastrephid squid paralarvae collected in northern Hawaiian waters and phylogenetic implications for the family Ommastrephidae using mtDNA analysis. Fish Sci 72, 494–502 (2006). https://doi.org/10.1111/j.1444-2906.2006.01177.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1444-2906.2006.01177.x