-
PDF
- Split View
-
Views
-
Cite
Cite
Miyoung Yoon, Andy Nong, Harvey J. Clewell, Michael D. Taylor, David C. Dorman, Melvin E. Andersen, Evaluating Placental Transfer and Tissue Concentrations of Manganese in the Pregnant Rat and Fetuses after Inhalation Exposures with a PBPK Model, Toxicological Sciences, Volume 112, Issue 1, November 2009, Pages 44–58, https://doi.org/10.1093/toxsci/kfp198
Close - Share Icon Share
Abstract
A Physiologically Based Pharmaco Kinetic (PBPK) model, based on a published description of manganese (Mn) kinetics in adult rats, has been developed to describe Mn uptake and tissue distribution in the pregnant dam and fetus during dietary and inhalation exposures. This extension incorporated key physiological processes controlling Mn pharmacokinetics during pregnancy and fetal development. After calibration against tissue Mn concentrations observed during late gestation, the model accurately simulated Mn tissue distribution in the dam and fetus following both diet and inhalation exposures to the pregnant rat. Maternal to fetal transfer of Mn through placenta was described using two pathways: a saturable active transport with high affinity and a simple diffusion. The active transport dominates at basal and lower Mn exposure, whereas at higher Mn exposure, the relative contribution of the diffusion pathway increases. To simulate fetal tissue Mn, tissue-binding parameters and preferential influx/efflux rates in fetal brain were adjusted from the adult model based on differential developmental processes and varying tissue demands for Mn in early life. Model simulations were consistent with observed tissue Mn concentrations in fetal tissues, including brain for diet alone and for combined diet and inhalation. Simulations of Mn in placenta and other maternal tissues in late gestation correlated well with measured tissue concentrations. This model, together with our published models for Mn kinetics during lactation and postnatal development, will help to address concerns about Mn neurotoxicity in potentially sensitive human subpopulation, such as infants and children by providing an estimate of Mn exposure in the population of interest.
Mn, an essential trace element, is required in variety of enzymatic and cellular process in the body (Roth, 2006). Yet, excess Mn is toxic to the central nervous system (CNS), with adverse responses most commonly found in occupational situations where workers may be exposed to extremely high atmospheric concentrations of Mn (Roth, 2006). Mn neurotoxicity in humans is characterized as a form of parkinsonism, Parkinson-like movement disorders preceded by neuropsychiatric symptoms (Aschner et al., 2005). These responses are correlated with selective accumulation of Mn in target brain regions, primarily in those areas known to control fine motor movement, including the striatum and globus pallidus (Aschner et al., 2005).
Normally, Mn concentrations are tightly maintained by homeostatic mechanisms controlling uptake, storage, and elimination, thereby protecting the body from wide fluctuations in daily dietary Mn exposures (Teeguarden et al., 2007). However, tissue accumulation of Mn may occur when the homeostatic mechanisms are overwhelmed by excessive Mn exposures and lead to CNS toxicity. A recently developed PBPK model for Mn in adult rats and monkeys successfully simulated Mn kinetics across doses from adequacy to excess. These models included dietary and inhalation exposures and differential control processes at work for these two routes of administration (Nong et al., 2009). The key processes included in the models were saturable tissue-binding kinetics, asymmetrical flux into tissues that accumulate Mn, induction of biliary excretion, and variable dietary absorption depending on the Mn content in foodstuffs (Nong et al., 2009). With these control processes descriptions, the tissue compartments retain fairly constant Mn during periods of normal dietary intake while allowing for rapid increases in tissue Mn levels when challenged with excessive Mn such as high-dose inhalation and rapid tissue clearance after the cessation of overexposure.
Mn is also required in the growing fetus to ensure normal development and in the dam to maintain maternal health. As with other essential elements, the challenge during the gestation and lactation is to balance fetal/neonatal demands and maternal homeostasis. Several biological and physiological changes during gestation and lactation periods compensate for losses from the dam to the fetus-dependent/lactational-dependent pups. Pregnancy and lactation lead to increased food intake (Shirley, 1984). General gastrointestinal function changes can allow greater absorptive capacity for many elements (Cripps and Williams, 1975). Often, these general changes are not enough to maintain sufficiency of the metal in both the developing offspring and the dam and more specific compensation may be necessary; for instance, a direct enhancement of gut absorption for the nutrient (Segués et al., 1987), probably at the level of specific transporters.
Essential elements are believed to be efficiently distributed from the maternal side to fetal circulation across the placenta. Several metal transporters including DMT1 are known to be expressed in the placenta and appear to participate in the transfer (Leazer and Klaassen, 2003). Hanlon et al. (1975) reported that Mn crosses the placenta and suggested the absence of any selective storage or barrier effect by the placenta for Mn under conditions of normal exposure. In addition, an experiment using multitracer techniques demonstrated that the placenta may serve as a filter and block uptake of nonessential metals by accumulating them in the placenta itself (Enomoto and Hirunuma, 2001). For essential metals such as Mn, the authors observed rapid uptake of the transferred Mn into fetal brain.
Concerns have been raised regarding increased accumulation of inhaled Mn in the fetal/neonatal brain due to higher demands for Mn during development. Rodent studies of enhanced CNS uptake during development have been contradictory. One indicated that the amount of Mn that crosses the placenta was not increased by enhanced maternal exposure via diet (Jarvinen and Ahlstrom, 1975). Dorman et al. (2005) also did not observe any increase in brain Mn concentration in fetuses from the dam exposed to Mn by inhalation during pregnancy. However, another study indicated increased neonatal brain Mn following chronic high-level exposure of the dam to Mn in drinking water throughout gestation (Kontur and Fechter, 1985).
To address the possibility of enhanced Mn uptake in the brain tissue during development, it is necessary to understand the CNS dosimetry of Mn in fetuses and pups for various dietary and inhalation exposure situations. Our goal here was to develop a PBPK model that could predict fetal Mn dose and Mn disposition in the dam and fetus following maternal exposures to Mn. The ultimate purpose was to create an integrated gestation/lactation model to improve our understanding of potential risks of Mn exposure by inhalation during intrauterine and postnatal developments (Yoon et al., forthcoming).
METHODS
Study Design and Data
The data used to parameterize the gestation model of Mn inhalation are mainly from Dorman et al. (2005). Although other studies were also used and influenced model development (Supplementary Data), many parameters had to be estimated from this single study due to lack of tissue Mn measurements in other Mn inhalation studies. Thus, this current work serves primarily to demonstrate the model development process for gestation/pregnancy, showing the extension of an adult model to a different life stage and assessing the critical biological/physiological processes required to describe Mn kinetics during gestation.
The present model simulates Mn inhalation exposures in the rat during gestation. The exposures were simulated for the study with Sprague-Dawley rats on a 10-ppm diet (Dorman et al., 2005). The inhalation exposure schedule was either air controls or manganese sulfate (at concentrations of 0.05, 0.5, or 1 mg Mn/m3) for 6 h/day, 7 days/week.
The exposure began 28 days before breeding and continued through up to a 14-day mating period; pregnancy exposure started from gestation day (GD) 0 to 20. Maternal and fetal tissue Mn concentrations were determined at the end of the last 6-h exposure on GD20. Since the tissue Mn concentrations in the dam were already in steady state after 28 days of inhalation during prebreeding, model predictions were similar for exposure durations of up to 14 days during the mating period. Manganese sulfate inhalation resulted in increased Mn concentration in several maternal tissues, including lung, brain, and placenta. Fetal brain Mn concentrations remained constant despite high concentration Mn exposure to the dam by inhalation (Dorman et al., 2005). Inhaled Mn concentrations in the Dorman et al. (2005) study were much higher than those observed in the environment, actually better representing exposures of workers at high levels in some mining or welding operations (Clewell et al., 2003a). There was no evidence of fetotoxicity at any of the inhalation concentrations utilized in the study with the rats. Gestation duration was modeled as 22 days; thus, dams of another cohort were kept without exposure for 2 days before giving birth on what was designated as postnatal day 0 (PND0). Predictions at the end of gestation from this model were utilized as initial values in simulating Mn exposure in a model of lactation (Yoon et al., forthcoming).
Model Structure
Modeling the Pregestation Period
Because the experimental methodology had a preconception period, the present model simulated Mn inhalation exposure initiated before conception. The end of simulation values from a preconception simulation were used as the GD0 parameter values. The preconception model is essentially the same as the adult model, except that the mammary gland was included for the adult female rats. Detailed descriptions including the parameter values used for simulating this period are in Supplementary Data.
Gestation Model
The Mn gestation model structure was based on the published adult rat model (Nong et al., 2009). The same approach for describing saturable tissue binding and brain diffusion of Mn was applied both for the dam and for the fetuses. The placenta and mammary gland were explicitly added to the model that already had lung, nose, brain, bone, liver, and a lumped body compartment, which includes all other tissues in the dam (Fig. 1). Three regions of brain that included with a brain-blood compartment were described in the dam. A litter of fetuses was modeled as one large aggregated fetal compartment for simplification since interlitter variability was not considered in the current model (Clewell et al., 2003b). The purpose of the model was to provide an estimate of average fetal dose based on available data from Dorman et al. (2005), in which fetal tissue Mn data were collected from several pooled fetuses from each litter. Due to small sample volume available from GD20 fetuses, the measurement of Mn in an individual fetus was not possible. The fetus includes four explicit compartments—lung, brain, bone, and liver—and a lumped compartment for the rest of body. In the fetus, whole brain was the unit of measure for the CNS rather than the separate regions in the adult model. Model parameters were abbreviated using subscripts and were given in parenthesis as necessary; X indicates either dam or fetus, D represents dam, and F indicates fetal parameter. AcslX version 2.4 (AEgis Technologies Group, Inc., Huntsville, AL) was used to run all the simulations. Model code in CSL file format is provided in the Supplementary Data.
Model structure for simulating Mn exposure during gestation in the rat. Mn present in the body either as free (Mnf) or bound form (Mnb). Mn binding capacity in the tissue is represented as ‘B’. Subscript ‘_F’ was used to distinguish fetal parameters from those of the dam. Fetal blood circulation was modeled as separate from that of the dam. Maternal Mn is transferred to the fetuses through the placenta in the free form. Refer to “Methods” section for other abbreviations.
The Mn exposure to the dam was either through diet or simultaneously through diet and inhalation. As in the adult, uptake of Mn from diet was described with a constant rate of transfer (KdietD) into the liver based on Mn concentration in food and daily food consumption rate. Only a certain portion of dietary Mn was absorbed into the body (FdietUpD). Mn was eliminated through bile (KbileD).
For inhalation, Mn particles are deposited onto the lung, nasal respiratory, and nasal olfactory epithelium after inhalation based on fractional deposition in each of these sites, i.e., fDepLu, fDepNR, and fDepNO, respectively. While most deposited Mn on the nasal epithelium was absorbed into systemic blood (nasal respiratory region), a small portion of deposited Mn (nasal olfactory region) was transferred directly to the olfactory bulb. The fate of Mn particles deposited in the lung was either absorption into the lung tissue or transfer to the systemic blood.
Fetal Mn exposure was through the placenta. Placental transfer was described as a bidirectional transfer process, between free Mn (Mnf) in placental tissue and fetal blood (Table 1). Mn flux from placenta to fetal blood was mediated by two separate pathways operating simultaneously; one was a bidirectional diffusion process and the other was an active transport mechanism with high affinity. Hence, maternal to fetal flux was described using a rate of diffusion (ktrans1C), a maximum velocity for the active transport (VmaxC), and a Michaelis-Menten constant, Km (Table 1). The placenta does not have binding capacity in the model. Fetal to maternal transfer from fetal blood to placental tissue was described using a first-order diffusion rate constant (ktrans2C) (Table 1).
Mn-Specific Parameter Values in the Dam and Fetuses
| Parameters for Mn tissue binding characteristics | ||||||
| Tissue binding | Brain diffusion | |||||
| BmaxCa | Ka | Kd | KinC | KoutC | PC | |
| Tissue | μg × kg−1 | μg−1 × h−1 | h−1 | h−1 × kg0.25 | h−1 × kg0.25 | tissue:blood |
| Pregnant dam | ||||||
| Bone | 605 | 0.18 | 0.024 | 0.5 | ||
| Brain | 1 | |||||
| Cerebellum | 690 | 1.32 | 0.00013 | 1 | 8 | |
| Olfactory bulb | 1200 | 0.87 | 0.00028 | 0.087 | 1.22 | |
| Striatum | 1500 | 0.026 | 0.001 | 1.7 | 1 | |
| Liver | 3100 | 0.28 | 0.011 | 0.85 | ||
| Lung | 9200 | 0.25 | 1.5 | 1 | ||
| Mammary gland | 200 | 0.093 | 0.0046 | 1 | ||
| Placenta | na | na | na | 0.45 | ||
| Rest of body | 200 | 0.093 | 0.0046 | 1 | ||
| Fetuses | ||||||
| Bone | 1984 | 0.18 | 0.024 | 1 | ||
| Brain | 540 | 0.03 | 0.001 | 2.55 | 1.5 | 1 |
| Liver | 1000 | 0.018 | 0.011 | 1 | ||
| Lung | 950 | 0.02 | 0.38 | 1 | ||
| Rest of body | 400 | 0.0093 | 0.0046 | 1 | ||
| Parameters for Mn tissue binding characteristics | ||||||
| Tissue binding | Brain diffusion | |||||
| BmaxCa | Ka | Kd | KinC | KoutC | PC | |
| Tissue | μg × kg−1 | μg−1 × h−1 | h−1 | h−1 × kg0.25 | h−1 × kg0.25 | tissue:blood |
| Pregnant dam | ||||||
| Bone | 605 | 0.18 | 0.024 | 0.5 | ||
| Brain | 1 | |||||
| Cerebellum | 690 | 1.32 | 0.00013 | 1 | 8 | |
| Olfactory bulb | 1200 | 0.87 | 0.00028 | 0.087 | 1.22 | |
| Striatum | 1500 | 0.026 | 0.001 | 1.7 | 1 | |
| Liver | 3100 | 0.28 | 0.011 | 0.85 | ||
| Lung | 9200 | 0.25 | 1.5 | 1 | ||
| Mammary gland | 200 | 0.093 | 0.0046 | 1 | ||
| Placenta | na | na | na | 0.45 | ||
| Rest of body | 200 | 0.093 | 0.0046 | 1 | ||
| Fetuses | ||||||
| Bone | 1984 | 0.18 | 0.024 | 1 | ||
| Brain | 540 | 0.03 | 0.001 | 2.55 | 1.5 | 1 |
| Liver | 1000 | 0.018 | 0.011 | 1 | ||
| Lung | 950 | 0.02 | 0.38 | 1 | ||
| Rest of body | 400 | 0.0093 | 0.0046 | 1 | ||
| Parameters determining placental transfer of Mn | ||||||
| Ktrans1C (l × h−1 × kg0.75)b | 0.001 | Diffusion rate constant scalar for describing Mn transfer from maternal placental tissue to fetal venous blood | ||||
| Ktrans2C (l × h−1 × kg0.75)b | 0.0004 | Diffusion rate constant scalar for describing Mn transfer from fetal arterial blood to maternal placental tissue | ||||
| VmaxC (μg × h−1 × kg0.75)b | 2 | Maximum velocity scalar for describing active transport from maternal placental tissue to fetal venous blood | ||||
| Km (μg × l−1) | 0.1 | Michaelis-Menten constant for Mn active transport from placenta to fetus | ||||
| Parameters determining placental transfer of Mn | ||||||
| Ktrans1C (l × h−1 × kg0.75)b | 0.001 | Diffusion rate constant scalar for describing Mn transfer from maternal placental tissue to fetal venous blood | ||||
| Ktrans2C (l × h−1 × kg0.75)b | 0.0004 | Diffusion rate constant scalar for describing Mn transfer from fetal arterial blood to maternal placental tissue | ||||
| VmaxC (μg × h−1 × kg0.75)b | 2 | Maximum velocity scalar for describing active transport from maternal placental tissue to fetal venous blood | ||||
| Km (μg × l−1) | 0.1 | Michaelis-Menten constant for Mn active transport from placenta to fetus | ||||
| Parameters governing Mn uptake and excretion | ||||||
| FDietUpD | 0.0319 | Fractional absorption | ||||
| KbileCD (l/h/kg0.75) | 0.0028 | Biliary excretion scalar | ||||
| Kbile2D (l/h) | EXPOSUREc × kbile × KBInducc | Induced biliary excretion during inhalation. Total biliary excretion during exposure = Kbile + Kbile2 | ||||
| KBInduc indicates the degree of dose-dependent induction | ||||||
| Parameters governing Mn uptake and excretion | ||||||
| FDietUpD | 0.0319 | Fractional absorption | ||||
| KbileCD (l/h/kg0.75) | 0.0028 | Biliary excretion scalar | ||||
| Kbile2D (l/h) | EXPOSUREc × kbile × KBInducc | Induced biliary excretion during inhalation. Total biliary excretion during exposure = Kbile + Kbile2 | ||||
| KBInduc indicates the degree of dose-dependent induction | ||||||
Note. na, not applicable; PC, partition coefficient.
Maximum binding capacity as μg Mn per kg tissue.
Scaled to individual fetal BW.
Indicates exposure duration. Biliary induction was modeled only during inhalation period.
Mn-Specific Parameter Values in the Dam and Fetuses
| Parameters for Mn tissue binding characteristics | ||||||
| Tissue binding | Brain diffusion | |||||
| BmaxCa | Ka | Kd | KinC | KoutC | PC | |
| Tissue | μg × kg−1 | μg−1 × h−1 | h−1 | h−1 × kg0.25 | h−1 × kg0.25 | tissue:blood |
| Pregnant dam | ||||||
| Bone | 605 | 0.18 | 0.024 | 0.5 | ||
| Brain | 1 | |||||
| Cerebellum | 690 | 1.32 | 0.00013 | 1 | 8 | |
| Olfactory bulb | 1200 | 0.87 | 0.00028 | 0.087 | 1.22 | |
| Striatum | 1500 | 0.026 | 0.001 | 1.7 | 1 | |
| Liver | 3100 | 0.28 | 0.011 | 0.85 | ||
| Lung | 9200 | 0.25 | 1.5 | 1 | ||
| Mammary gland | 200 | 0.093 | 0.0046 | 1 | ||
| Placenta | na | na | na | 0.45 | ||
| Rest of body | 200 | 0.093 | 0.0046 | 1 | ||
| Fetuses | ||||||
| Bone | 1984 | 0.18 | 0.024 | 1 | ||
| Brain | 540 | 0.03 | 0.001 | 2.55 | 1.5 | 1 |
| Liver | 1000 | 0.018 | 0.011 | 1 | ||
| Lung | 950 | 0.02 | 0.38 | 1 | ||
| Rest of body | 400 | 0.0093 | 0.0046 | 1 | ||
| Parameters for Mn tissue binding characteristics | ||||||
| Tissue binding | Brain diffusion | |||||
| BmaxCa | Ka | Kd | KinC | KoutC | PC | |
| Tissue | μg × kg−1 | μg−1 × h−1 | h−1 | h−1 × kg0.25 | h−1 × kg0.25 | tissue:blood |
| Pregnant dam | ||||||
| Bone | 605 | 0.18 | 0.024 | 0.5 | ||
| Brain | 1 | |||||
| Cerebellum | 690 | 1.32 | 0.00013 | 1 | 8 | |
| Olfactory bulb | 1200 | 0.87 | 0.00028 | 0.087 | 1.22 | |
| Striatum | 1500 | 0.026 | 0.001 | 1.7 | 1 | |
| Liver | 3100 | 0.28 | 0.011 | 0.85 | ||
| Lung | 9200 | 0.25 | 1.5 | 1 | ||
| Mammary gland | 200 | 0.093 | 0.0046 | 1 | ||
| Placenta | na | na | na | 0.45 | ||
| Rest of body | 200 | 0.093 | 0.0046 | 1 | ||
| Fetuses | ||||||
| Bone | 1984 | 0.18 | 0.024 | 1 | ||
| Brain | 540 | 0.03 | 0.001 | 2.55 | 1.5 | 1 |
| Liver | 1000 | 0.018 | 0.011 | 1 | ||
| Lung | 950 | 0.02 | 0.38 | 1 | ||
| Rest of body | 400 | 0.0093 | 0.0046 | 1 | ||
| Parameters determining placental transfer of Mn | ||||||
| Ktrans1C (l × h−1 × kg0.75)b | 0.001 | Diffusion rate constant scalar for describing Mn transfer from maternal placental tissue to fetal venous blood | ||||
| Ktrans2C (l × h−1 × kg0.75)b | 0.0004 | Diffusion rate constant scalar for describing Mn transfer from fetal arterial blood to maternal placental tissue | ||||
| VmaxC (μg × h−1 × kg0.75)b | 2 | Maximum velocity scalar for describing active transport from maternal placental tissue to fetal venous blood | ||||
| Km (μg × l−1) | 0.1 | Michaelis-Menten constant for Mn active transport from placenta to fetus | ||||
| Parameters determining placental transfer of Mn | ||||||
| Ktrans1C (l × h−1 × kg0.75)b | 0.001 | Diffusion rate constant scalar for describing Mn transfer from maternal placental tissue to fetal venous blood | ||||
| Ktrans2C (l × h−1 × kg0.75)b | 0.0004 | Diffusion rate constant scalar for describing Mn transfer from fetal arterial blood to maternal placental tissue | ||||
| VmaxC (μg × h−1 × kg0.75)b | 2 | Maximum velocity scalar for describing active transport from maternal placental tissue to fetal venous blood | ||||
| Km (μg × l−1) | 0.1 | Michaelis-Menten constant for Mn active transport from placenta to fetus | ||||
| Parameters governing Mn uptake and excretion | ||||||
| FDietUpD | 0.0319 | Fractional absorption | ||||
| KbileCD (l/h/kg0.75) | 0.0028 | Biliary excretion scalar | ||||
| Kbile2D (l/h) | EXPOSUREc × kbile × KBInducc | Induced biliary excretion during inhalation. Total biliary excretion during exposure = Kbile + Kbile2 | ||||
| KBInduc indicates the degree of dose-dependent induction | ||||||
| Parameters governing Mn uptake and excretion | ||||||
| FDietUpD | 0.0319 | Fractional absorption | ||||
| KbileCD (l/h/kg0.75) | 0.0028 | Biliary excretion scalar | ||||
| Kbile2D (l/h) | EXPOSUREc × kbile × KBInducc | Induced biliary excretion during inhalation. Total biliary excretion during exposure = Kbile + Kbile2 | ||||
| KBInduc indicates the degree of dose-dependent induction | ||||||
Note. na, not applicable; PC, partition coefficient.
Maximum binding capacity as μg Mn per kg tissue.
Scaled to individual fetal BW.
Indicates exposure duration. Biliary induction was modeled only during inhalation period.
As in the adult model (Nong et al., 2009), Mn in the tissue is either free or bound. The binding is reversible, with a specified binding capacity (Bmaxtissuex) that is tissue specific. The rate and extent of binding in each tissue are determined by association and dissociation rate constants (Katissuex and Kdtissuex, respectively) together with Bmaxtissuex. Mn concentration in the brain regions was described with region-specific influx and efflux rates (KinregionD and KoutregionD, respectively). For fetal brain, only one set of KinF and KoutF was used to describe the whole brain.
Although the basic concepts and structure of the adult model did not change, some modifications were necessary to accommodate different life stages such as pregnancy and fetal development in rats (Table 1). These modifications were made only when sufficient rationale were available based on known pharmacokinetic behavior changes during gestation, either specifically for Mn or for essential elements in general if information is not available for Mn. The modification of the adult model structure was performed sequentially due to limited data availability. First, control group data were used to estimate gestation-specific parameters under normal dietary condition and then refined by adjusting inhalation exposure-specific changes appropriately for pregnancy and fetal development periods. The parameter values were optimized manually to obtain a reasonable fit to Mn concentrations in all tissues. A flow chart illustrating this sequential process of the model development and parameterization is provided in Supplementary Data along with the citations for the data and references that influenced the decision process (Supplementary Data).
Model Parameterization
Mn-Specific Parameters
Mn-specific model parameters are in Table 1. Descriptions of the changes are also provided if model parameters were changed for pregnancy or fetal growth compared to the adult model (Table 1).
Nonpregnant Female
Parameters for simulating nonpregnant female adults in the preconception period are given in Supplementary Data.
Pregnant Dam
Uptake and elimination.
Adult values for fractional uptake (FdietUpD) and biliary excretion scalar (KbileCD) for a 10-ppm diet were used for nonexposed, pregnant dam (Nong et al., 2009). For a definition for the term scalar, refer to the Supplementary Data. With high-concentration inhalation exposures, biliary elimination was appropriately induced for the pregnant dam. With these changes, the simulated placental concentrations and fetal tissue concentrations agree with the observations of Dorman et al. (2005). Biliary induction occurred at Mn doses at 0.5 and 1 mg/m3 by adding an induced biliary excretion rate (kbile2D) to the basal excretion rate (kbileD). The extent of induction was dependent on Mn dose. The inclusion of biliary induction was appropriate since Mn inhalation duration from pregestation through gestation was consistent with exposures increasing biliary excretion in the adult rat model (Nong et al., 2009).
Mn particle deposition.
Mn particle deposition in the dam from inhalation was simulated using the Multiple Path Particle Dosimetry model software version 2.0 (Anjilvel and Asgharian, 1995; The Hamner Institutes, http://www.thehamner.org/technology-and-development/technology-transfer) based on the adult model (Nong et al., 2009). A particle size of 1.05 μm (geometric mean of count median diameter), density of 2.95 g/cm3, and geometric SD of 1.5 were utilized in the simulation (Dorman et al., 2005). Fractional deposition in nasal olfactory region was calculated as previously described for adults assuming olfactory epithelium deposition being proportional to olfactory airflow (Kelly et al., 2001; Kimbell et al., 1997; Nong et al., 2009). Fractional deposition of Mn particles (as manganese sulfate) in nasal respiratory, nasal olfactory, and pulmonary regions were included in the model as 0.412, 0.073, and 0.136, respectively.
Parameters for tissue binding and partition.
For mammary gland, tissue-binding parameters such as the maximum binding capacity (BmaxC, per unit tissue weight) and the association and dissociation rate constants and partition coefficient (PC) for rest of body compartment were used in the pregnant dam (Table 1). The PC for the mammary gland was also the same as rest of body (Table 1). The placenta was described without a specific Mn binding capacity. The placenta:blood Mn PC was lower than those for other compartments, reflecting a lower placental concentration as compared to maternal blood (Dorman et al., 2005).
Values for tissue maximum binding capacity in the dam (Table 1) were adjusted to fit the model to observed data for nonexposed animals during gestation (Dorman et al., 2005). Liver Mn levels were higher in the pregnant rat than nonpregnant female (Jarvinen and Ahlstrom, 1975). Maximum binding capacity in maternal liver was increased proportionately to account for this difference (Table 1).
Fetuses
Parameters for Mn tissue binding.
Both the BmaxCF (per unit tissue weight) and the tissue binding rates (KaF) were adjusted to simulate fetal concentrations (Table 1). These changes likely reflect differential developmental processes for various tissues, differing degree of storage capacity, and Mn incorporation rates, i.e., demands for Mn in the individual tissues. BmaxCF for fetal lung and liver were reduced compared to adults, while higher values of BmaxCF were used for bone and rest of the body compartments. Bone is considered as an Mn storage site during prenatal development (Enomoto and Hirunuma, 2001). The increment in BmaxCF of rest of body was based on the observation that zinc concentration in fetal skeletal muscle was much higher than the maternal tissue suggesting a potential role of the muscle as another storage site for essential elements during intrauterine life (Simmer et al., 1985). Amnionic fluid was not included in the present model based on the experimental data showing no Mn in the fluid when Mn tracer was injected in the dam (Enomoto and Hirunuma, 2001).
Parameters for influx/efflux in brain.
Whole brain was modeled for the fetal compartment. Consistent with a less mature blood-brain barrier (BBB) function in fetal brain, the influx rate constant (KinCF) from brain blood to brain tissue was slightly increased compared to the adult striatal value. The efflux rate constant (KoutCF) was also increased. In this way, Mn passes through BBB more freely in the fetus than in adults (Table 1). However, the ratio of Kin/Kout was still higher than the adult, i.e., influx was more preferred in the fetus compared to adults.
Estimation of Mn Placental Transfer Rates
Mn transfer through the placenta was described as a bidirectional exchange, between Mnf in placental tissue and fetal blood. The two components of Mn transport from the dam to fetus were included in the model based on the information known for the mechanisms and regulations of fetoplacental transport of essential metals (Leazer and Klaassen, 2003; McArdle et al., 2008; Stulc and Stulcová, 1986). The placental transport of essential elements is highly asymmetrical, maternal-fetal transport primarily through active transport mechanism(s), with only a small bidirectional flux via diffusion (Simmer et al., 1985; Stulc and Stulcová, 1986). This situation also appears applicable for Mn placental transport as shown by a rapid transfer of Mn tracer from the placenta to fetus in pregnant rat (Enomoto and Hirunuma, 2001) and by Mn tracer disposition in maternal tissues and the embryo of a Syrian hamster (Hanlon et al., 1975).
The rate constants and affinity constant for placental transfer processes in the absence of inhalation were parameterized using two data sets (Dorman et al., 2005; Kostial et al., 2005). VmaxC and Km were used for describing maternal to fetal active transport, ktrans1C for the diffusion rate in the same direction, and ktrans2C for fetal to maternal diffusion. First, these parameters were estimated by assessing whether the simulated body Mn concentration and amount in the fetal whole body fell within the ranges observed in newborn Wistar rats whose mothers were on a diet containing sufficient Mn (Kostial et al., 2005) (Supplementary Data). Preliminary values were refined against the placental and fetal tissue concentrations on GD20 reported in Dorman et al. (2005).
The Vmax and Km of the active transporter were set so that the process is working almost at saturation levels during normal pregnancy with sufficient Mn in diet (Table 1). Two other findings supports active transport: (1) the iron transfer to the fetus against a concentration gradient even in maternal iron deficiency (McArdle et al., 2008) and (2) the observed high affinity of the maternal-fetal transport system for calcium in rat placenta (Stulc and Stulcová, 1986).
Maternal to fetal transfer is dominant. However, other evidence shows that the reverse direction of movement also occurs, i.e., the fetal to maternal direction, albeit with a lower contribution to total transport (Simmer et al., 1985; Stulc and Stulcová, 1986). The scalar for maternal to fetal diffusion rate constant (ktrans1C) was set to fivefold greater than the scalar for reverse direction transfer rate (fetal to maternal, ktrans2C) to match the observed placental and fetal tissue data (Dorman et al., 2005).
The parameters for placental transfer were scaled by fetal body weight0.75 (BW), including ktrans1C, VmaxC, and ktrans2C (Table 1). Placental transfer rate of essential elements is believed to be proportional to fetal size. The increase in placental transport is also believed to be related to the maturation of the placenta (Dorman et al., 2001a).
Once the basal placental transfer rates were reasonably well parameterized against the data (Dorman et al., 2005; Kostial et al., 2005), the diffusion rate constants were refined based on Mn tissue concentrations in the inhalation exposures (Dorman et al., 2005). The inclusion of both the active transport and the diffusion produces efficient Mn transport to the fetus at basal state, with small increases in Mn transfer occurring under inhalation exposure. Hence, the active transport process dominates in the basal state, but under inhalation exposures, active transport becomes saturated and the relative role of diffusion increases (Table 1).
Physiological Parameters
Physiological parameters are provided in Table 2 along with the sources from which they were obtained or modified. The equations describing growth or changing parameters during gestation are listed in Supplementary Data. Data for Sprague-Dawley rats were selected for incorporation in the model whenever possible. Data collected from nonanesthetized rats were the preferred sources. Priority was given to the individual data set from one study rather than using the compilation data and to the data that cover a broader period of gestation.
Physiological Parameters Used For Pregnant Dam and Fetuses
| Parameters | References | |||
| Pregnant dam | ||||
| BW, kg | 0.24–0.38 | Clewell et al. (2003b); Dorman et al. (2005) | ||
| Cardiac output index (QCI, l × h−1 × kg−1)a | 24.5–21.6 | Dowell and Kauer (1997) | ||
| Alveolar ventilation index (QPI, l × h−1 × kg−1)a | 23.5–19.9 | Leavens et al. (2006) | ||
| Tissue volume (fraction of BW0) | ||||
| Blood | 0.0676 | Brown et al. (1997) | ||
| Bone | 0.073 | Brown et al. (1997) | ||
| Brain | 0.006 | Brown et al. (1997) | ||
| Liver | 0.04 | Buelke-Sam et al. (1982) | ||
| Lung | 0.0042 | Buelke-Sam et al. (1982) | ||
| Brain regional volume (as fraction of brain volume) | ||||
| Brain blood | 0.03 | Assumption | ||
| Cerebellum | 0.12 | Dorman et al. (2001b) | ||
| Olfactory bulb | 0.03 | Dorman et al. (2001b) | ||
| Striatum | 0.04 | Dorman et al. (2001b) | ||
| Tissue volume (l, actual volume, not changing during pregnancy) | ||||
| Nose | Calculated from surface area and thickness of the nasal tissue | |||
| Surface area (cm2 × kg−0.75) | Respiratory nasal cavity | 19.74 | Menache et al. (1997) | |
| Olfactory nasal cavity | 25.16 | Menache et al. (1997) | ||
| Average thickness nasal cavity (μm) | 84 | Conolly et al. (2000) | ||
| Tissue volume (l, actual volume, changing during pregnancy) | ||||
| Mammary gland (VM) | 0.0024–0.013 | Hanwell and Linzell (1973); Rosso et al. (1981) | ||
| Fat (VF)b | 0.017–0.024 | Brown et al. (1997); Naismith et al. (1982) | ||
| Placenta (Vpla, for a whole litter) | 0–0.0167 | Clewell et al. (2003b); O’Flaherty et al. (1992) | ||
| Rest of body | Difference between BW and the sum of the other tissue volumes | |||
| Tissue blood flow (fraction of initial cardiac output, QC0) | ||||
| Bone | 0.122 | Brown et al. (1997) | ||
| Brain | 0.02 | Brown et al. (1997) | ||
| Liver | 0.2408 | Brown et al. (1997) | ||
| Nose | 0.01 | Schroeter et al. (2008) | ||
| Tissue blood flow (l/h, actual flow changing during pregnancy) | ||||
| Mammary gland (QM) | 0.012–0.064 | Clewell et al. (2003b); Hanwell and Linzell (1973) | ||
| Placenta (Qpla, for a whole litter) | 0–1.42 | Clewell et al. (2003b); O’Flaherty et al. (1992) | ||
| Rest of body | Difference between cardiac output and the sum of the other tissue blood flows except lung | |||
| Food consumption | ||||
| Food intake (g × day−1 × kg−1) | TABLE, 68–84 | Shirley (1984) | ||
| Fetus | ||||
| BW (V1fet, kg) | 0–0.0068 | Sikov and Thomas (1970) | ||
| Cardiac output Index (QCI_F, l × h−1 × kg−1) | 22.8 | Girard et al. (1983) | ||
| Tissue volume (fraction of BW) | ||||
| Blood | 0.0676 | Brown et al. (1997) | ||
| Bone | 0.073 | Brown et al. (1997) | ||
| Tissue volume (l, growth equation for actual volume) | ||||
| Brain | 0–0.0034 | Sikov and Thomas (1970) | ||
| Liver | 0–0.0044 | Sikov and Thomas (1970) | ||
| Lung | 0–0.0019 | Sikov and Thomas (1970) | ||
| Tissue volume (actual volume) | ||||
| Rest of body (l) | Difference between BW and the sum of the other tissue volumes | |||
| Tissue blood flow (fraction of cardiac output) | ||||
| Bone | 0.122 | Brown et al. (1997) | ||
| Brain | 0.1055 | Carter and Gu (1988) | ||
| Liver | 0.061 | Itskovitz et al. (1987) | ||
| Lung | 0.08 | Itskovitz et al. (1987) | ||
| Rest of body | Difference between cardiac output and the sum of the other tissue blood flows | |||
| Parameters | References | |||
| Pregnant dam | ||||
| BW, kg | 0.24–0.38 | Clewell et al. (2003b); Dorman et al. (2005) | ||
| Cardiac output index (QCI, l × h−1 × kg−1)a | 24.5–21.6 | Dowell and Kauer (1997) | ||
| Alveolar ventilation index (QPI, l × h−1 × kg−1)a | 23.5–19.9 | Leavens et al. (2006) | ||
| Tissue volume (fraction of BW0) | ||||
| Blood | 0.0676 | Brown et al. (1997) | ||
| Bone | 0.073 | Brown et al. (1997) | ||
| Brain | 0.006 | Brown et al. (1997) | ||
| Liver | 0.04 | Buelke-Sam et al. (1982) | ||
| Lung | 0.0042 | Buelke-Sam et al. (1982) | ||
| Brain regional volume (as fraction of brain volume) | ||||
| Brain blood | 0.03 | Assumption | ||
| Cerebellum | 0.12 | Dorman et al. (2001b) | ||
| Olfactory bulb | 0.03 | Dorman et al. (2001b) | ||
| Striatum | 0.04 | Dorman et al. (2001b) | ||
| Tissue volume (l, actual volume, not changing during pregnancy) | ||||
| Nose | Calculated from surface area and thickness of the nasal tissue | |||
| Surface area (cm2 × kg−0.75) | Respiratory nasal cavity | 19.74 | Menache et al. (1997) | |
| Olfactory nasal cavity | 25.16 | Menache et al. (1997) | ||
| Average thickness nasal cavity (μm) | 84 | Conolly et al. (2000) | ||
| Tissue volume (l, actual volume, changing during pregnancy) | ||||
| Mammary gland (VM) | 0.0024–0.013 | Hanwell and Linzell (1973); Rosso et al. (1981) | ||
| Fat (VF)b | 0.017–0.024 | Brown et al. (1997); Naismith et al. (1982) | ||
| Placenta (Vpla, for a whole litter) | 0–0.0167 | Clewell et al. (2003b); O’Flaherty et al. (1992) | ||
| Rest of body | Difference between BW and the sum of the other tissue volumes | |||
| Tissue blood flow (fraction of initial cardiac output, QC0) | ||||
| Bone | 0.122 | Brown et al. (1997) | ||
| Brain | 0.02 | Brown et al. (1997) | ||
| Liver | 0.2408 | Brown et al. (1997) | ||
| Nose | 0.01 | Schroeter et al. (2008) | ||
| Tissue blood flow (l/h, actual flow changing during pregnancy) | ||||
| Mammary gland (QM) | 0.012–0.064 | Clewell et al. (2003b); Hanwell and Linzell (1973) | ||
| Placenta (Qpla, for a whole litter) | 0–1.42 | Clewell et al. (2003b); O’Flaherty et al. (1992) | ||
| Rest of body | Difference between cardiac output and the sum of the other tissue blood flows except lung | |||
| Food consumption | ||||
| Food intake (g × day−1 × kg−1) | TABLE, 68–84 | Shirley (1984) | ||
| Fetus | ||||
| BW (V1fet, kg) | 0–0.0068 | Sikov and Thomas (1970) | ||
| Cardiac output Index (QCI_F, l × h−1 × kg−1) | 22.8 | Girard et al. (1983) | ||
| Tissue volume (fraction of BW) | ||||
| Blood | 0.0676 | Brown et al. (1997) | ||
| Bone | 0.073 | Brown et al. (1997) | ||
| Tissue volume (l, growth equation for actual volume) | ||||
| Brain | 0–0.0034 | Sikov and Thomas (1970) | ||
| Liver | 0–0.0044 | Sikov and Thomas (1970) | ||
| Lung | 0–0.0019 | Sikov and Thomas (1970) | ||
| Tissue volume (actual volume) | ||||
| Rest of body (l) | Difference between BW and the sum of the other tissue volumes | |||
| Tissue blood flow (fraction of cardiac output) | ||||
| Bone | 0.122 | Brown et al. (1997) | ||
| Brain | 0.1055 | Carter and Gu (1988) | ||
| Liver | 0.061 | Itskovitz et al. (1987) | ||
| Lung | 0.08 | Itskovitz et al. (1987) | ||
| Rest of body | Difference between cardiac output and the sum of the other tissue blood flows | |||
Scaled to the actual changing BW during pregnancy, not the BW0.
This is not for a description as a separate compartment. Fat volume was used for calculating maternal BW change purpose only.
Physiological Parameters Used For Pregnant Dam and Fetuses
| Parameters | References | |||
| Pregnant dam | ||||
| BW, kg | 0.24–0.38 | Clewell et al. (2003b); Dorman et al. (2005) | ||
| Cardiac output index (QCI, l × h−1 × kg−1)a | 24.5–21.6 | Dowell and Kauer (1997) | ||
| Alveolar ventilation index (QPI, l × h−1 × kg−1)a | 23.5–19.9 | Leavens et al. (2006) | ||
| Tissue volume (fraction of BW0) | ||||
| Blood | 0.0676 | Brown et al. (1997) | ||
| Bone | 0.073 | Brown et al. (1997) | ||
| Brain | 0.006 | Brown et al. (1997) | ||
| Liver | 0.04 | Buelke-Sam et al. (1982) | ||
| Lung | 0.0042 | Buelke-Sam et al. (1982) | ||
| Brain regional volume (as fraction of brain volume) | ||||
| Brain blood | 0.03 | Assumption | ||
| Cerebellum | 0.12 | Dorman et al. (2001b) | ||
| Olfactory bulb | 0.03 | Dorman et al. (2001b) | ||
| Striatum | 0.04 | Dorman et al. (2001b) | ||
| Tissue volume (l, actual volume, not changing during pregnancy) | ||||
| Nose | Calculated from surface area and thickness of the nasal tissue | |||
| Surface area (cm2 × kg−0.75) | Respiratory nasal cavity | 19.74 | Menache et al. (1997) | |
| Olfactory nasal cavity | 25.16 | Menache et al. (1997) | ||
| Average thickness nasal cavity (μm) | 84 | Conolly et al. (2000) | ||
| Tissue volume (l, actual volume, changing during pregnancy) | ||||
| Mammary gland (VM) | 0.0024–0.013 | Hanwell and Linzell (1973); Rosso et al. (1981) | ||
| Fat (VF)b | 0.017–0.024 | Brown et al. (1997); Naismith et al. (1982) | ||
| Placenta (Vpla, for a whole litter) | 0–0.0167 | Clewell et al. (2003b); O’Flaherty et al. (1992) | ||
| Rest of body | Difference between BW and the sum of the other tissue volumes | |||
| Tissue blood flow (fraction of initial cardiac output, QC0) | ||||
| Bone | 0.122 | Brown et al. (1997) | ||
| Brain | 0.02 | Brown et al. (1997) | ||
| Liver | 0.2408 | Brown et al. (1997) | ||
| Nose | 0.01 | Schroeter et al. (2008) | ||
| Tissue blood flow (l/h, actual flow changing during pregnancy) | ||||
| Mammary gland (QM) | 0.012–0.064 | Clewell et al. (2003b); Hanwell and Linzell (1973) | ||
| Placenta (Qpla, for a whole litter) | 0–1.42 | Clewell et al. (2003b); O’Flaherty et al. (1992) | ||
| Rest of body | Difference between cardiac output and the sum of the other tissue blood flows except lung | |||
| Food consumption | ||||
| Food intake (g × day−1 × kg−1) | TABLE, 68–84 | Shirley (1984) | ||
| Fetus | ||||
| BW (V1fet, kg) | 0–0.0068 | Sikov and Thomas (1970) | ||
| Cardiac output Index (QCI_F, l × h−1 × kg−1) | 22.8 | Girard et al. (1983) | ||
| Tissue volume (fraction of BW) | ||||
| Blood | 0.0676 | Brown et al. (1997) | ||
| Bone | 0.073 | Brown et al. (1997) | ||
| Tissue volume (l, growth equation for actual volume) | ||||
| Brain | 0–0.0034 | Sikov and Thomas (1970) | ||
| Liver | 0–0.0044 | Sikov and Thomas (1970) | ||
| Lung | 0–0.0019 | Sikov and Thomas (1970) | ||
| Tissue volume (actual volume) | ||||
| Rest of body (l) | Difference between BW and the sum of the other tissue volumes | |||
| Tissue blood flow (fraction of cardiac output) | ||||
| Bone | 0.122 | Brown et al. (1997) | ||
| Brain | 0.1055 | Carter and Gu (1988) | ||
| Liver | 0.061 | Itskovitz et al. (1987) | ||
| Lung | 0.08 | Itskovitz et al. (1987) | ||
| Rest of body | Difference between cardiac output and the sum of the other tissue blood flows | |||
| Parameters | References | |||
| Pregnant dam | ||||
| BW, kg | 0.24–0.38 | Clewell et al. (2003b); Dorman et al. (2005) | ||
| Cardiac output index (QCI, l × h−1 × kg−1)a | 24.5–21.6 | Dowell and Kauer (1997) | ||
| Alveolar ventilation index (QPI, l × h−1 × kg−1)a | 23.5–19.9 | Leavens et al. (2006) | ||
| Tissue volume (fraction of BW0) | ||||
| Blood | 0.0676 | Brown et al. (1997) | ||
| Bone | 0.073 | Brown et al. (1997) | ||
| Brain | 0.006 | Brown et al. (1997) | ||
| Liver | 0.04 | Buelke-Sam et al. (1982) | ||
| Lung | 0.0042 | Buelke-Sam et al. (1982) | ||
| Brain regional volume (as fraction of brain volume) | ||||
| Brain blood | 0.03 | Assumption | ||
| Cerebellum | 0.12 | Dorman et al. (2001b) | ||
| Olfactory bulb | 0.03 | Dorman et al. (2001b) | ||
| Striatum | 0.04 | Dorman et al. (2001b) | ||
| Tissue volume (l, actual volume, not changing during pregnancy) | ||||
| Nose | Calculated from surface area and thickness of the nasal tissue | |||
| Surface area (cm2 × kg−0.75) | Respiratory nasal cavity | 19.74 | Menache et al. (1997) | |
| Olfactory nasal cavity | 25.16 | Menache et al. (1997) | ||
| Average thickness nasal cavity (μm) | 84 | Conolly et al. (2000) | ||
| Tissue volume (l, actual volume, changing during pregnancy) | ||||
| Mammary gland (VM) | 0.0024–0.013 | Hanwell and Linzell (1973); Rosso et al. (1981) | ||
| Fat (VF)b | 0.017–0.024 | Brown et al. (1997); Naismith et al. (1982) | ||
| Placenta (Vpla, for a whole litter) | 0–0.0167 | Clewell et al. (2003b); O’Flaherty et al. (1992) | ||
| Rest of body | Difference between BW and the sum of the other tissue volumes | |||
| Tissue blood flow (fraction of initial cardiac output, QC0) | ||||
| Bone | 0.122 | Brown et al. (1997) | ||
| Brain | 0.02 | Brown et al. (1997) | ||
| Liver | 0.2408 | Brown et al. (1997) | ||
| Nose | 0.01 | Schroeter et al. (2008) | ||
| Tissue blood flow (l/h, actual flow changing during pregnancy) | ||||
| Mammary gland (QM) | 0.012–0.064 | Clewell et al. (2003b); Hanwell and Linzell (1973) | ||
| Placenta (Qpla, for a whole litter) | 0–1.42 | Clewell et al. (2003b); O’Flaherty et al. (1992) | ||
| Rest of body | Difference between cardiac output and the sum of the other tissue blood flows except lung | |||
| Food consumption | ||||
| Food intake (g × day−1 × kg−1) | TABLE, 68–84 | Shirley (1984) | ||
| Fetus | ||||
| BW (V1fet, kg) | 0–0.0068 | Sikov and Thomas (1970) | ||
| Cardiac output Index (QCI_F, l × h−1 × kg−1) | 22.8 | Girard et al. (1983) | ||
| Tissue volume (fraction of BW) | ||||
| Blood | 0.0676 | Brown et al. (1997) | ||
| Bone | 0.073 | Brown et al. (1997) | ||
| Tissue volume (l, growth equation for actual volume) | ||||
| Brain | 0–0.0034 | Sikov and Thomas (1970) | ||
| Liver | 0–0.0044 | Sikov and Thomas (1970) | ||
| Lung | 0–0.0019 | Sikov and Thomas (1970) | ||
| Tissue volume (actual volume) | ||||
| Rest of body (l) | Difference between BW and the sum of the other tissue volumes | |||
| Tissue blood flow (fraction of cardiac output) | ||||
| Bone | 0.122 | Brown et al. (1997) | ||
| Brain | 0.1055 | Carter and Gu (1988) | ||
| Liver | 0.061 | Itskovitz et al. (1987) | ||
| Lung | 0.08 | Itskovitz et al. (1987) | ||
| Rest of body | Difference between cardiac output and the sum of the other tissue blood flows | |||
Scaled to the actual changing BW during pregnancy, not the BW0.
This is not for a description as a separate compartment. Fat volume was used for calculating maternal BW change purpose only.
Nonpregnant Female
Physiological parameters for simulating nonpregnant female adults for describing the pregestation period are in Supplementary Data.
Pregnant Dam
Maternal BW at the beginning of gestation (prepregnancy) was obtained from Dorman et al. (2005). The increase in maternal BW was calculated as the sum of changing tissue volumes during gestation and the initial prepregnancy body weight, i.e., initial body weight on GD0. This approach was a modification from Clewell et al. (2003b), where increasing volume of the placenta, mammary gland, fat, and total fetal weight were noted to be mostly responsible for the BW increase. All other maternal tissue volumes were described as constant fractions of initial body weight (BW0), with volumes remaining constant throughout gestation. Fractional tissue volumes for blood, brain, and bone were adapted from Brown et al. (1997) and liver and lung values were taken from Buelke-Sam et al. (1982) reported for nonpregnant CD colony of rats at NCTR. Although fat was not included as a separate compartment in the model structure, the increase in fat volume was accounted for in the calculation of the total BW of the pregnant dam.
The volume of the mammary gland linearly increased from 0.01% (Hanwell and Linzell, 1973), reaching 3.73% of total BW of the dam on GD21 (Rosso et al., 1981); this approach leads to a mammary gland volume of 5% of postbirth BW (i.e., maternal body weight excluding placenta and fetal body weight) at the end of gestation. The change in fat volume was also described as a linear increase during gestation, assuming a 40% increase (Naismith et al., 1982) from the volume of 7% BW for nonpregnant Sprague-Dawley rat (Brown et al., 1997).
Brain region volumes as a fraction of total brain volume for striatum, cerebellum, and olfactory bulb were kept constant during gestation, and the same fractional volumes were used as in the adult (Nong et al., 2009). Surface area and tissue thickness of the olfactory and respiratory regions of the nose in maternal rats were similar to the adult model using the same scalars (Nong et al., 2009) with the dam initial BW, assuming no volume changes during gestation for these nasal regions.
Individual placental volume per conceptus was described as a sum of the yolk sac and chorioallantonic placenta (Clewell et al., 2003b); the description of the growth and decline of each of the two individual components of the placenta was adapted from O’Flaherty et al. (1992). The placental volume increased beginning at GD6 (O’Flaherty et al., 1992). The remaining body volume was calculated as the difference between the BW and the summed volume of the maternal tissue compartments and the total fetal volume.
Alveolar ventilation during gestation was simulated using a linearly decreasing ventilation scalar (l/h/kg) determined in pregnant Sprague-Dawley rats (Leavens et al., 2006). Cardiac output (QC) in the pregnant dam was modeled using a scalar (cardiac index [QCI], l/h/kg BW) based on reported cardiac output changes during gestation for conscious Sprague-Dawley rats (Dowell, 1997). Initial cardiac output (QC0) on GD0, calculated by multiplying initial body weight (BW0) and QCI value on GD0 (QCI0), was then used for describing blood flow to the tissues whose volumes did not change during gestation.
The fraction of maternal cardiac output to tissues such as liver, brain, bone, and nose that does not grow during gestation was determined by multiplying the fractional blood flow scalar for nonpregnant or GD0 rats by QC0, so that tissue blood flow (l/h) remains constant during gestation. Fractional blood flows for these tissues in nonpregnant or GD0 female rats were obtained from Brown et al. (1997) for the brain, bone, and liver and from Nong et al. (2009) for the nose. In simulating the fractional blood flow to the liver, flow through the hepatic artery and the portal flow from the gastrointestinal tract were combined.
Blood flow to the mammary gland was proportional to the mammary gland volume (Clewell et al., 2003b). Initial mammary flow on GD0 was adapted from Hanwell and Linzell (1973). The fraction of maternal cardiac output to the placenta was a combination of blood flow changes to yolk sac placenta and chorioallantonic placenta during pregnancy (Clewell et al., 2003b; O’Flaherty et al., 1992). Blood flow to the remaining body compartments was described by subtracting combined flows to brain, bone, liver, nose, and mammary gland from total cardiac output.
Maternal food intake during gestation came from the data for Sprague-Dawley rats (Shirley, 1984) and was incorporated into the simulations with a TABLE function.
Fetuses
Thirteen fetuses were modeled per litter based on the average litter size for Sprague-Dawley rats as reported in a Mn inhalation study during gestation (Dorman et al., 2005). The fetal compartment was a single compartment for an entire litter. A Gompertz curve simulated increases in individual fetus BW during gestational development based on fetal growth data given by Sikov and Thomas (1970), the main data sets used by O’Flaherty et al. (1992). The fetal growth curve used in the current model was chosen to avoid discontinuities in model predictions due to the discontinuity in the previous fetal growth description (O’Flaherty et al., 1992).
The volumes of fetal liver, lung, and brain were simulated using Gompertz equations based on those given by Sikov and Thomas (1970) for Sprague-Dawley rat fetuses during late gestation, from GD14 to birth. These curves were also used to extrapolate fetal tissue volumes before GD14. Since the information was not available for bone and blood volume in rat fetus, the relative volumes of these two tissues (as fraction of fetal BW) were held constant over the period of fetal development using the same fractional volumes as in adults (Brown et al., 1997). The remaining body compartment in the fetus was calculated as the difference between the fetal BW and the sum of the other tissues.
Fetal blood circulation, a separate system working independently from the maternal blood system, increased with fetal growth (Clewell et al., 2003b). Cardiac output reported in fetal guinea pig was utilized in the present rat model (Girard et al., 1983). The relative tissue blood flows for liver and lung in the fetus with respect to its BW were adapted from fetal sheep data in late gestation (Itskovitz et al., 1987). The distribution of fetal cardiac output reported in sheep was similar to that for the guinea pig (Girard et al., 1983). Data for cardiac output and fractional blood flow to liver and lung in rat fetuses were not available. Cerebral blood flow for the fetal rat in late gestation was 1.07 ml/min/g brain (Carter and Gu, 1988). The relative blood flow to fetal brain in the model was adjusted to produce fetal brain flow per unit tissue mass reaching this reported value at the end of gestation on GD22. Since there are no data available for fractional blood flow to fetal bone, the same fraction was utilized as in adults (Brown et al., 1997).
Model Predictions
End of Exposure Tissue Concentration
Maternal and fetal tissue concentrations on GD20 immediately after the cessation of the last inhalation exposure were simulated (Figs. 2–4) for each Mn inhalation exposure concentrations. The simulated tissue Mn concentrations were compared to the observed data in Dorman et al. (2005). For brain Mn, whole-brain concentration was measured for the dam and fetus (Dorman et al., 2005). Since the current model used regional brain description in the dam, simulated cerebellum concentration was selected as a surrogate for comparison to the whole-brain concentration in the dam. For fetal Mn, whole-brain concentration was simulated and compared to the whole-brain Mn seen in the fetus (Dorman et al., 2005).
Simulation of placental Mn concentrations on GD20. Solid line represents simulated placental concentrations on GD20 at each Mn inhalation concentration. Placental concentrations reported in Dorman et al. (2005) are shown by mean ± SEM.
Simulation of maternal tissue Mn concentrations on GD20. Solid line represents model simulated (A) liver, (B) cerebellum brain, and (C) bone Mn concentrations in the dam. Respective tissue concentration reported in Dorman et al. (2005) were mean ± SEM. For comparison of the brain concentration, simulation was for cerebellum, while the data represent whole brain.
Simulation of fetal tissue Mn concentrations on GD20. Solid line represents simulated (A) liver, (B) brain, and (C) bone Mn concentrations in the fetus. Respective tissue concentration reported in Dorman et al. (2005) were represented by mean ± SEM.
Although model predictions were not made for earlier GDs, it was necessary to describe time-dependent accumulation of Mn in the fetus in order to predict GD20 values since Mn is already present in the body. Description of Mn kinetics during early gestation was based upon several assumptions based on the current understanding of Mn distribution. However, we were reluctant to make any predictions for earlier gestation due to the greater uncertainties with respect to both physiology and Mn biology during this period and the lack of experimental data to evaluate model predictions at these early times.
Calculation of Daily Internal Dose Metrics
As a measure of the internal dose metric, a 24-h cumulative area under the curve (24-h AUC) for Mn concentrations in cerebellum was calculated as a daily internal dose metric for maternal brain. For the fetus, the 24-h AUC for Mn concentration in whole brain was calculated. The daily AUC (24-h AUC) on GD20 was calculated by subtracting the AUC from GD0 to GD20 from the AUC from GD0 to GD21.
Amount of Mn Transferred to Fetuses
Mn daily dose to the fetus on GD20 was determined using the difference between the amount of Mn transferred to the fetus from GD0 to GD20 and that from GD0 to GD21. Since there is a backward Mn flux from the fetus to the dam as well, the true amount of Mn transferred into the fetus was calculated by excluding the backward transfer amount from fetus to the dam (Atrans2) from the amount of Mn transferred from the maternal placenta to fetus (Atrans1).
Prediction of Mn Concentration at Birth
One of the objectives of the present model was to provide initial conditions (e.g., gestational carry over) to an Mn lactation model (Yoon et al., forthcoming) in order to simulate the outcome of combined in utero and lactational exposures. Hence, the end of simulation values on GD22, which is designated as the end of gestation and the birth, at each Mn inhalation dose were predicted and utilized as the PND0 parameter values for the lactation model (Yoon et al., forthcoming). Outputs used for this purpose were both the free and the bound Mn in tissues. Since the fetal model used whole-brain description, the amount in the whole brain was appropriately proportioned based on the relative volume of each brain region and utilized as initial amount in the corresponding brain region in the neonates.
Model Evaluation
The adequacy of the model parameter values was evaluated visually following the same criteria applied in the lactation model (Yoon et al., forthcoming). The correspondence between the predicted values and experimental data (Dorman et al., 2005; Kostial et al., 2005) was evaluated, and the goodness of the model prediction in the present model was considered to be reasonable.
Sensitivity and Uncertainty Analyses
The impact of parameter uncertainty on model predictions was evaluated semiquantitatively through a combination of sensitivity analysis and a qualitative evaluation of parameter uncertainty following the approach used in Teeguarden et al. (2005). The purpose of this analysis was to identify the key parameters that can add uncertainty in the fetal brain Mn internal dose metrics, further research on which will be able to contribute to better understanding of fetal brain Mn.

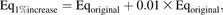
where Eq represents an equation used to describe growth or a changing process during simulation.
Sensitivity analysis was performed for control (Mn 0 mg/m3) or inhalation (Mn 0.5 mg/m3) group, examining the impact of model parameters on the concentration of total Mn in fetal brain (CtotBrnF) or AUCbrainF for total Mn in fetal brain under normal dietary exposure or Mn inhalation as well as dietary exposure on GD20.
The relative influence of each of the model parameters on simulated fetal brain Mn concentration and AUC on GD20 was examined based on the calculated SCs. All the model parameters were subject to the sensitivity analysis and those with SC greater than 0.2 were reported (Table 3). The complete results of the sensitivity analysis are reported in Supplementary Data. Each model parameter was categorized as having low, medium, or high impact on the selected fetal brain Mn dose metric with the following criteria:
Low (L): SC < 0.2.
Medium (M): 0.2 ≤ SC < 0.5.
High (H): 0.5 ≤ SC.
Gestation Model Sensitivity and Uncertainty Designations
| Late gestation (GD20) | ||||||
| Fetal brain Mn concentration/fetal brain Mn AUC | ||||||
| SC | Sensitivity designation | |||||
| Parameters | Control | Inhalation | Control | Inhalation | Uncertainty designation | |
| Mn placental transfer | Active transport (Vmax) | 0.48/0.51 | 0.43/0.49 | H/H | H/H | M |
| Fetal parameters | ||||||
| Physiological parameters | BW (V1fet) | −0.21/−0.21 | −0.19/−0.20 | M/M | M/M | L |
| Volume of brain (VbrainF) | 0.26/0.26 | 0.23/0.25 | M/M | M/M | L | |
| Mn tissue-binding/uptake parameters | Bmax in brain (BmaxbrainF) | 0.95/0.70 | 0.95/0.69 | H/H | H/H | H |
| Bmax in other tissues (BmaxOthF) | −0.21/a | a/a | M/L | M/L | M | |
| Influx rate into brain (KinbrainF) | 0.28/0.39 | 0.25/0.39 | M/M | M/M | M | |
| Efflux rate from brain (KoutbrainF) | −0.27/−0.39 | −0.25/−0.38 | M/M | M/M | M | |
| Association rate in brain (KabrainF) | 0.25/0.21 | 0.22/0.20 | M/M | M/M | M | |
| Mn tissue partition coefficients | Brain PC (PbrainF) | 0.28/0.49 | 0.26/0.49 | M/M | M/M | H |
| Other tissues PC (POthF) | a/−0.30 | a/−0.29 | L/M | L/M | H | |
| Late gestation (GD20) | ||||||
| Fetal brain Mn concentration/fetal brain Mn AUC | ||||||
| SC | Sensitivity designation | |||||
| Parameters | Control | Inhalation | Control | Inhalation | Uncertainty designation | |
| Mn placental transfer | Active transport (Vmax) | 0.48/0.51 | 0.43/0.49 | H/H | H/H | M |
| Fetal parameters | ||||||
| Physiological parameters | BW (V1fet) | −0.21/−0.21 | −0.19/−0.20 | M/M | M/M | L |
| Volume of brain (VbrainF) | 0.26/0.26 | 0.23/0.25 | M/M | M/M | L | |
| Mn tissue-binding/uptake parameters | Bmax in brain (BmaxbrainF) | 0.95/0.70 | 0.95/0.69 | H/H | H/H | H |
| Bmax in other tissues (BmaxOthF) | −0.21/a | a/a | M/L | M/L | M | |
| Influx rate into brain (KinbrainF) | 0.28/0.39 | 0.25/0.39 | M/M | M/M | M | |
| Efflux rate from brain (KoutbrainF) | −0.27/−0.39 | −0.25/−0.38 | M/M | M/M | M | |
| Association rate in brain (KabrainF) | 0.25/0.21 | 0.22/0.20 | M/M | M/M | M | |
| Mn tissue partition coefficients | Brain PC (PbrainF) | 0.28/0.49 | 0.26/0.49 | M/M | M/M | H |
| Other tissues PC (POthF) | a/−0.30 | a/−0.29 | L/M | L/M | H | |
Represents an SC smaller than 0.2.
Gestation Model Sensitivity and Uncertainty Designations
| Late gestation (GD20) | ||||||
| Fetal brain Mn concentration/fetal brain Mn AUC | ||||||
| SC | Sensitivity designation | |||||
| Parameters | Control | Inhalation | Control | Inhalation | Uncertainty designation | |
| Mn placental transfer | Active transport (Vmax) | 0.48/0.51 | 0.43/0.49 | H/H | H/H | M |
| Fetal parameters | ||||||
| Physiological parameters | BW (V1fet) | −0.21/−0.21 | −0.19/−0.20 | M/M | M/M | L |
| Volume of brain (VbrainF) | 0.26/0.26 | 0.23/0.25 | M/M | M/M | L | |
| Mn tissue-binding/uptake parameters | Bmax in brain (BmaxbrainF) | 0.95/0.70 | 0.95/0.69 | H/H | H/H | H |
| Bmax in other tissues (BmaxOthF) | −0.21/a | a/a | M/L | M/L | M | |
| Influx rate into brain (KinbrainF) | 0.28/0.39 | 0.25/0.39 | M/M | M/M | M | |
| Efflux rate from brain (KoutbrainF) | −0.27/−0.39 | −0.25/−0.38 | M/M | M/M | M | |
| Association rate in brain (KabrainF) | 0.25/0.21 | 0.22/0.20 | M/M | M/M | M | |
| Mn tissue partition coefficients | Brain PC (PbrainF) | 0.28/0.49 | 0.26/0.49 | M/M | M/M | H |
| Other tissues PC (POthF) | a/−0.30 | a/−0.29 | L/M | L/M | H | |
| Late gestation (GD20) | ||||||
| Fetal brain Mn concentration/fetal brain Mn AUC | ||||||
| SC | Sensitivity designation | |||||
| Parameters | Control | Inhalation | Control | Inhalation | Uncertainty designation | |
| Mn placental transfer | Active transport (Vmax) | 0.48/0.51 | 0.43/0.49 | H/H | H/H | M |
| Fetal parameters | ||||||
| Physiological parameters | BW (V1fet) | −0.21/−0.21 | −0.19/−0.20 | M/M | M/M | L |
| Volume of brain (VbrainF) | 0.26/0.26 | 0.23/0.25 | M/M | M/M | L | |
| Mn tissue-binding/uptake parameters | Bmax in brain (BmaxbrainF) | 0.95/0.70 | 0.95/0.69 | H/H | H/H | H |
| Bmax in other tissues (BmaxOthF) | −0.21/a | a/a | M/L | M/L | M | |
| Influx rate into brain (KinbrainF) | 0.28/0.39 | 0.25/0.39 | M/M | M/M | M | |
| Efflux rate from brain (KoutbrainF) | −0.27/−0.39 | −0.25/−0.38 | M/M | M/M | M | |
| Association rate in brain (KabrainF) | 0.25/0.21 | 0.22/0.20 | M/M | M/M | M | |
| Mn tissue partition coefficients | Brain PC (PbrainF) | 0.28/0.49 | 0.26/0.49 | M/M | M/M | H |
| Other tissues PC (POthF) | a/−0.30 | a/−0.29 | L/M | L/M | H | |
Represents an SC smaller than 0.2.
The uncertainty of the model parameters with medium or high impact on the selected dose metric was evaluated qualitatively. The emphasis was put on the availability of the data for the gestation in the case of physiological parameters, and the availability of the information for Mn during fetal growth and pregnancy for chemical-specific parameters. The uncertainty was designated as follows:
Low (L): Data were directly available for the parameter or the value was optimized using the relevant tissue Mn data.
Medium (M): Information on pharmacokinetics of Mn available and/or underlying biological processes relevant for the parameter available for Mn.
High (H): Data were not directly available for Mn. Reasonable assumptions can be made based on the information on other essential elements or related biological processes relevant for the parameter description or all others.
RESULTS
Placenta and Maternal Tissue Mn
The simulated Mn concentrations in placenta on GD20 at the end of the last exposure were consistent with the experimental observation (Fig. 2). Maternal liver, bone, and cerebellum concentrations on GD20 immediately after the end of the exposure were simulated and compared to the available data (Fig. 3). Accurate prediction of maternal tissue and placenta Mn concentrations increases confidence in the simulated tissue concentrations in the fetus. The present model was able to simulate dose-dependent changes in placenta and maternal tissue concentrations reasonably well. In the case of maternal whole-brain Mn, comparisons were made to predicted cerebella concentration in the dam (Fig. 3). The rise in striatum or in olfactory bulb under inhalation exposure is likely to differ from changes in whole-brain concentration, which may partly explain some inconsistencies between the simulated and observed brain concentrations seen at high inhaled concentrations (Fig. 3B).
Fetal Tissue Mn
The simulated brain concentration at the end of exposure on GD20 was consistent with the data either with or without Mn inhalation. The present model was consistent with the observation that fetal brain Mn concentration was not particularly sensitive to Mn inhalation exposures in the dam (Fig. 4B). Simulations for fetal liver and bone Mn concentrations on GD20 were also close to measured data. Total amount of Mn in the fetus at the end of gestation without inhalation exposure (Supplementary Data) was in a good agreement with the amount reported in newborn Wistar rats (Kostial et al., 2005).
Comparison of Maternal and Fetal Exposures
Risks to the fetus depend on the amount of chemical that passes from the placenta to the fetus and the resulting fetal exposure to the chemical. To compare internal exposures between the mother and fetus, 24-h AUC for whole brain (or cerebellum in the case of the dam) or blood (μg × h/g) on GD20 were plotted (Fig. 5). The exposure level in the fetal brain was lower than in equivalent tissues in the dam at all doses. The cerebellar concentrations, used for maternal brain daily AUC calculation, were slightly underestimated compared to the maternal whole brain. Thus, the daily AUC for fetal brain at higher Mn concentration would be even lower compared to the internal exposure of the dam. Blood 24-h AUCs also showed that the fetal internal exposures were lower or comparable to the dam. These simulations support the finding that Mn inhalation during gestation up to 1 mg/m3 did not increase fetal Mn exposure in the brain and consequently there was no noticeable dose-dependent increase in Mn exposure at the target tissue in the fetus.
Comparison of maternal and fetal exposures: daily AUC for brain or blood on GD20. Simulated maternal and fetal daily AUC on GD20 for brain or blood are compared. In the case of maternal brain, cerebellum concentration was used to calculate 24-h AUC.
Estimating Daily Mn Dose to the Fetus
The amount of Mn retained by the fetus during the 24-h period on GD20 was designated as daily Mn dose to the fetus. The estimated Mn dose to the individual fetus at each Mn concentration (Fig. 6) was virtually constant regardless of Mn inhaled dose to the dam.
Estimation of daily Mn dose to the fetus on GD20. Amounts of Mn transferred to the individual fetus during 24 h on GD20 were expressed as μg transferred to an individual fetus at each Mn exposure concentration to the dam.
Sensitivity and Uncertainty Analyses
The results for uncertainty analysis of the model parameters are presented in Table 3. The key parameters for fetal brain Mn exposure were similar between the control and exposed animals. The most sensitive parameters for fetal brain Mn were BmaxbrainF and Vmax for the active transport of Mn through the placenta. The relative importance of BmaxbrainF was greater in the control than inhalation group, while that of KinbrainF, KoutbrainF, and brain to blood Mn PC was higher in the inhalation group compared to the control (Table 3). The uncertainty designation for these parameters was either M or H, indicating that they can be considered to have potential to add uncertainty to model predicted fetal brain Mn internal dose metrics. Further studies on the placental transfer of Mn and the transport mechanism(s) of Mn in the brain as well as the biology of brain Mn during fetal development will be able to improve our understanding of potential risks of Mn neurotoxicity in the fetuses.
Physiological parameters such as BW and brain volume of the fetuses were also shown to be influential to fetal brain Mn. However, the confidence in the physiological parameters was considered high since the data are available for the parameters. Model predicted fetal brain Mn concentration or AUC was not sensitive to any of the maternal parameters (refer to Supplementary Data). This is not unexpected since all the placental transfer parameters were dependent on fetal growth.
DISCUSSION
The development and application of a complex simulation model for the alterations in basic physiology during pregnancy and lactation in the dam and during development of a fetus from single cell to a weaning is challenging by itself. With Mn, other physiological and biochemical processes had to be considered for the uptake and maintenance of tissue concentrations of this essential element to maintain the balance of Mn for the dam and the fetus—the two units that are in effect competing for the nutrient. Our philosophy was to develop the physiological description for pregnancy, lactation, and fetal/neonatal development and then utilize this basic structure, along with the adult Mn model to provide a starting point for simulating Mn concentrations in gestation, lactation, and postnatal life.
One of the key elements of assessing risks in potentially sensitive populations is to estimate an internal dose in target tissues in the susceptible population, which in the present study is fetal brain Mn. Our goal was to compare increases in brain Mn between the fetus and the adult and between the animals exposed by diet alone and those exposed through diet and inhalation. The present gestational PBPK model made these comparisons possible for Mn in relation to total brain Mn. It also supports estimation of the inhaled Mn concentrations that are expected to cause increases in fetal brain Mn compared to background levels.
In this regard, the model in its present state of development can provide important information for assessing risks of inhalation of Mn during gestation. The rat gestation model can be scaled to humans just as the adult rat Mn model has been scaled from rat to monkeys (Nong et al., 2009) and to the human model.
Our PBPK modeling effort has demonstrated that it is possible to produce simulations of brain Mn that are consistent with observed levels in late gestation by considering the physiology of gestation, developmental processes that affect Mn pharmacokinetics during intrauterine development, and control mechanisms of Mn homeostasis. Obviously, any PBPK model is a simplification of real biological system. Nong et al. (2008, 2009) described Mn behavior in the body based on saturable tissue binding and homeostatic control for its dietary uptake and biliary excretion. The consistency of this model structure across dose and for different animal species was demonstrated in the process of extrapolation of the model structure to the monkey (Nong et al., 2009). In the present study, we followed their approach by incorporating developmental changes for the processes that govern Mn tissue binding and control of its homeostasis in the body in conjunction with gestation physiology. Thus, we consider that the current model structure is consistent with the processes at work throughout other life stages and useful for estimating target tissue Mn exposure during the life stage of interest, i.e., gestation.
The present model was able to simulate Mn pharmacokinetic behaviors during gestation in the rat following maternal exposure both by diet only and by diet with inhalation (Dorman et al., 2005). An important contribution of this modeling effort is the enhanced understanding of the fundamental biological processes that govern the pharmacokinetics of Mn during gestation. In this regard, one of the most important features of this model is the description of the placental transfer process of Mn, which determines Mn dose to the fetus. The essence of this process is to ensure adequate Mn accretion to the fetuses allowing them to meet the higher demand for this element for proper development, while maintaining the maternal homeostasis to the extent possible. However, would this be a valid assumption when the dam is exposed to excessive amounts of Mn by inhalation? Would the fetus accumulate Mn due to the efficient transfer mechanism from the maternal body to the fetus? These questions needed to be answered to address the concern for potentially increased susceptibility toward Mn during fetal development following maternal exposure. Because deficiency during gestation is generally a concern for proper fetal development considering the essentiality of trace elements including Mn, less information is known regarding oversupply of this trace element or other essential elements. The success in predicting Mn concentrations in the pregnant dam and fetuses with or without inhalation demonstrated that the current model captures and appropriately incorporates key aspects of Mn kinetics during gestation both for basal and for overdose states.
Several studies showed a rapid and efficient transfer of essential elements through the placenta from maternal body to the fetus (Enomoto and Hirunuma, 2001; Simmer et al., 1985; Stulc and Stulcová, 1986), while transport of nonessential and/or toxic metals is restricted (Ahokas and Dilts, 1979; Enomoto and Hirunuma, 2001). The placental transfer of essential elements is thought to be largely mediated by metal transporters, including ZnT1, DMT1, CTR1, Menkes, and Wilsons (Leazer and Klaassen, 2003; McArdle et al., 2008). Upregulation of DMT1 during maternal iron deficiency results in an increased iron flux to the fetus minimizing potential anemia in the fetus (Gambling et al., 2001). Since many transporters are shared among essential elements, changes in the status of one element during pregnancy in the dam could cause an altered transport of other element (McArdle et al., 2008).
Higher needs of Mn in the fetus and neonates have raised concerns for increased risks to Mn overexposure during these early life stages. Indeed, rapid uptake of most of the Mn tracer transferred from the placenta into fetal brain was observed (Enomoto and Hirunuma, 2001). However, the results should be interpreted based on the essentiality and excess of essential elements. Although relatively few studies have been performed for excessive exposure conditions of the essential elements during pregnancy, several findings suggest that the resulting fetal concentration did not seem to be greatly influenced by increased maternal body burden up to a certain level. In vivo rodent studies, where Mn exposure of the dam occurs by the oral route, demonstrated that the amount of Mn crossing the placenta is relatively constant over a fairly wide range of Mn oral exposures (Jarvinen and Ahlstrom, 1975). However, fetal Mn concentration was sharply increased at extremely high levels of Mn in diet at which dose maternal homeostasis control is expected to be extremely disturbed (Jarvinen and Ahlstrom, 1975). In vitro experiments investigating rodent placental transporters for zinc and calcium showed that these transporters possess high affinity but saturable characteristics. Maternal to fetal transfer does not increase proportionally with increasing maternal concentration of these elements (Simmer et al., 1985; Stulc and Stulcová, 1986). Although only limited information is available regarding the distribution of Mn in fetal tissues after Mn inhalation exposure in the dam, the fetal brain did not accumulate Mn following maternal Mn inhalation up to 1 mg/m3 in the rat (Dorman et al., 2005). However, others observed increased neonatal brain Mn resulting from chronic overexposure of the dam to Mn throughout gestation via drinking water (Kontur and Fechter, 1985). These discrepancies are likely to be attributable to actual Mn dose to the fetus.
One of the main purposes of the present effort was to understand the inhalation exposure concentration that would disrupt maternal homeostasis to the extent that the fetal Mn exposure consequently increases. In the present model, the active transport system in the placenta was near saturation at basal state. With increasing maternal Mn body burden caused by inhalation exposure, this placental transfer path became saturated. Any increase in Mn flux into the fetus after the saturation of the active transport would be through a simple diffusion, which is not as efficient as the given active transport. Model simulations demonstrated that up to 1 mg/m3 inhalation concentration of Mn did not significantly increase the total amount of Mn transferred through placenta and consequently fetal brain exposure stayed comparable to that in the nonexposed fetuses. In addition, the comparison of Mn exposures between the dam and fetus suggests that fetal tissue exposures did not exceed that of the dam’s at any Mn inhaled concentrations.
Fechter (1999) summarized several findings on Mn distribution during gestation in rodents by comparing the total amount of Mn in fetal body to the placenta and/or maternal tissue concentrations. However, an increase in the placental concentration does not necessarily mean higher placental transfer of Mn to the fetus or higher fetal exposure (Dorman et al., 2005). Although placental concentration was significantly increased at > 0.5mg/m3 Mn inhaled dose, fetal brain concentrations were not increased (Dorman et al., 2005).
For simulating fetal tissue Mn, some of the model parameters were modified from adult values to appropriately describe developmental processes for different tissues during gestation. Brain, bone, and muscle (as part of rest of body compartment) were considered as high-demand tissues for Mn during fetal development. Tissue-binding and brain diffusion parameters were adjusted to ensure enough uptake of Mn into these tissues during gestation. Mn uptake rate into the fetus was reported to be higher compared to that into the placenta when an iv Mn tracer was given to the mother (Enomoto and Hirunuma, 2001). Furthermore, most of the tracer in the fetus was found in the brain reflecting higher demand for this tissue. Bone was considered to constitute a fetal store for Mn. Ribs of fetal pigs had a much higher concentration of Mn than other fetal tissues or the ribs of the dam (Widdowson et al., 1974). Mn found in skullcap in the fetus was at a higher concentration than the concentration in maternal femur (Dorman et al., 2005).
Both the immaturity of the BBB and increased demand during the organ’s development are considered responsible for higher uptake of Mn into fetal brain. Brain DMT1 and transferrin receptor, present in neonatal brain and known to be active with iron and zinc, are regarded as contributors of the putative Mn transport mechanisms as in adults (Erikson et al., 2007; Siddappa et al., 2002). However, little is known about transport mechanisms of Mn during intrauterine development. Maximal postnatal transferrin level in the rat brain was found immediately after birth; transferrin level is also found at high concentrations in the fetal CNS and cerebrospinal fluid (CSF). These observations indicate increased transport of Mn or other essential metals, including iron into fetal brain (Roskams and Connor, 1994). Transferrin in the fetal and early neonatal brain was considered to be from plasma and CSF. The BBB and blood-CSF barrier to plasma proteins are considered relatively leaky at early postnatal times (Dorman et al., 2001a), and transferrin messenger RNA in brain is expressed at its lowest level (Roskams and Connor, 1994).
Tissues that are not considered to have high-demand stage during gestation such as liver and lung were modeled with a lower maximum binding capacity and a smaller Ka. These adjustments likely reflect relatively lower Mn incorporation into these tissues during prenatal development compared to postnatal development period.
For some essential elements, the extent of fractional uptake in the gut is temporarily increased to maintain maternal homeostasis in the face of the high demand of these elements during pregnancy and lactation. The temporal patterns for this increase vary depending on the metal. In the case of iron, the gut absorption increases substantially during late gestation, which rapidly returns to basal level postpartum (Millard et al., 2004). On the other hand, the fraction of calcium absorbed from the gut starts to increase in late gestation, continues to be elevated during lactation, and then falls back to control values in the postweaning period (Halloran and DeLuca, 1980). These differences may reflect the differential demands for these elements during gestation and lactation. The parameterization required for Mn indicated that fractional uptake of Mn during pregnancy seemed to be similar to that of the adults while it appeared to be increased during lactation (Yoon et al., forthcoming). Liver was an exception; liver Mn levels were higher in the pregnant rat than in the nonpregnant female (Jarvinen and Ahlstrom, 1975). The liver may participate in handling increased Mn intakes resulting from the increased food intake during gestation (Jarvinen and Ahlstrom, 1975).
The present model predicted nearly constant concentrations of Mn in the placenta during gestation with a slight decrease in the late gestation period. This pattern was also observed for late gestation placental Mn in guinea pigs (Golub et al., 1996). The rapid growth of the fetuses during the late gestation period could contribute to this decrease in placental Mn concentration during late gestation.
We evaluated model performance using the only available data, i.e., the GD20 fetuses. Thus, it is not possible to determine the accuracy of the model for earlier periods of gestation. Verification for earlier times in gestation would require a different study design. However, prior to that effort, information needs to be collected on early gestation physiology and on the brain Mn in fetuses of different ages.
The model was successfully developed by incorporating key biological processes of placental transfer and differential development of fetal tissue development and achieved a good agreement between the observed data and model predictions. Fetal brain Mn was lower than that seen in the dam following Mn inhalation. The model also provided initial tissue Mn levels for simulating lactational exposure to Mn. Our successes describing Mn concentrations during gestation suggest that the present model together with the published lactation model will be able to predict the relationship between Mn tissue concentrations in target organ and inhalation exposure concentrations during different developmental stages. The ultimate goal of developing a PBPK model for Mn during gestation and lactation was to enhance the scientific basis for assessing the human health risks of Mn in potentially susceptive subpopulation by providing improved dose metrics in the target tissue during early life exposures.
FUNDING
Afton Chemical Corporation in satisfaction of registration requirements arising under Section 211(a) and (b) of the Clean Air Act and corresponding regulations at 40 Code of Federal Regulations Substance 79.50 et seq.
The authors would like to thank Drs. Hugh Barton, Jeffrey Fisher, Michele Medinsky, Daniel Krewski, Jerry Campbell Jr, and Rebecca Clewell for helpful comments during the preparation of this manuscript.
References



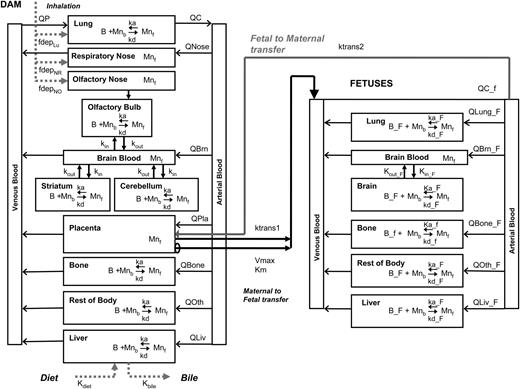
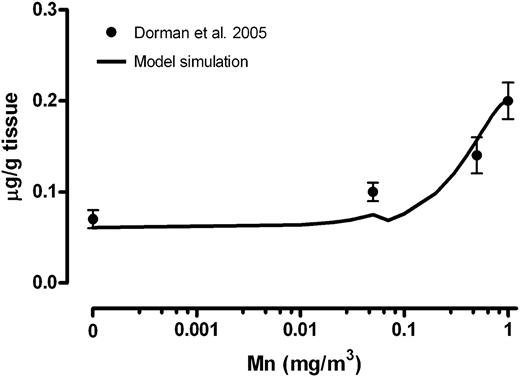
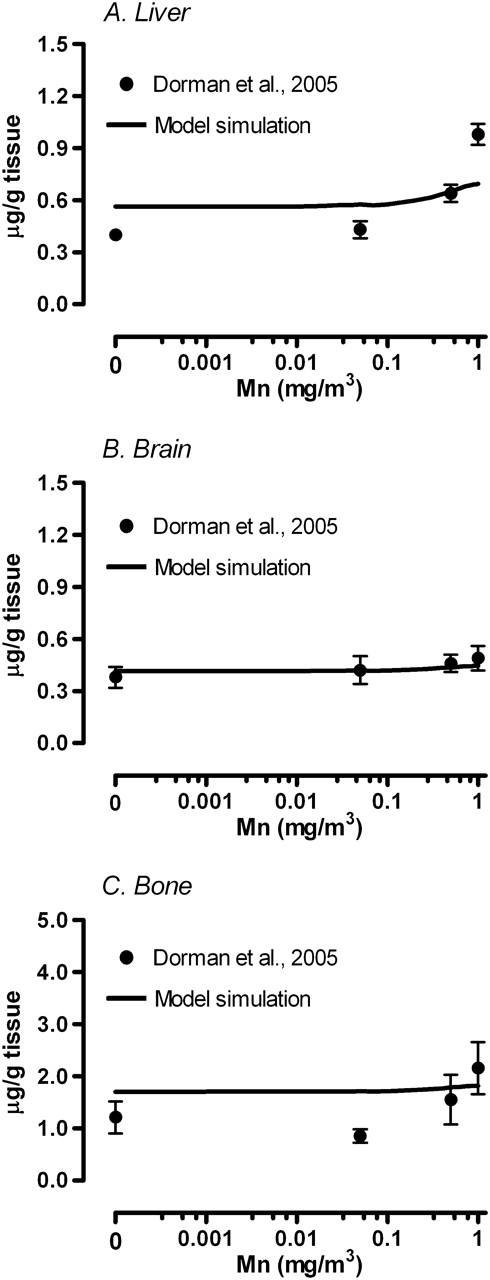
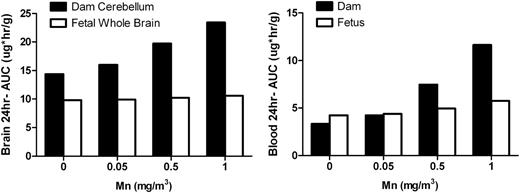
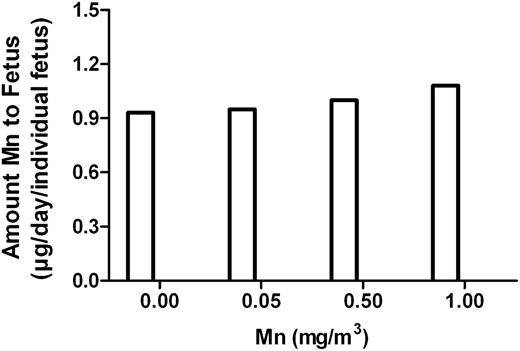

Comments