-
PDF
- Split View
-
Views
-
Cite
Cite
Donghong Gao, Jane Kasten-Jolly, David A. Lawrence, The Paradoxical Effects of Lead in Interferon-Gamma Knockout BALB/c Mice, Toxicological Sciences, Volume 89, Issue 2, February 2006, Pages 444–453, https://doi.org/10.1093/toxsci/kfj043
Close - Share Icon Share
Abstract
It has been reported that lead (Pb) exposure enhances interleukin (IL)-4 and inhibits interferon-gamma (IFNγ) production in wild-type (WT) BALB/c mice. Here, we examined Pb effects on immunity in IFNγ knockout (KO) mice. Lead significantly enhanced serum IgG1 anti-keyhole limpet hemocyanin (KLH) levels in WT mice compared to the controls; Pb also increased serum IgG2a anti-KLH levels, but the IgG1:IgG2a ratio was greater with Pb. In addition, total serum IgE levels, but not IgE anti-KLH levels, were increased. In the KO mice, the serum IgG1, IgG2a, IgE anti-KLH, and total IgE levels were significantly lower than those of WT mice. Surprisingly, Pb significantly enhanced IgG1and IgG2a anti-KLH levels in the KO mice. However, for these mice, unlike the WT mice, Pb caused a greater percentage change in IgG2a than in IgG1 anti-KLH, indicating less skewing toward type-2 immunoglobulins. Lead also enhanced the delayed-type hypersensitivity (DTH) response in WT mice. Not surprisingly, very low DTH occurred in the KO mice; however, Pb induced a strong KLH-specific DTH response. The in vivo Pb exposure significantly increased in vitro production of IL-4, IL-5, and IL-10, but not IFNγ, IL-2 and IL-12, by KLH-induced WT and KO spleen cells. In contrast to KLH, dinitrofluorobenzene contact hypersensitivity (DNFB CHS) was detected in all groups, and Pb did not affect this response, which suggests that Pb has only a slight effect on CD8+ T cell–related responses. As previously reported, Pb enhances Th2 responses in WT mice; however, in the KO mice, Pb enhanced Th1-related anti-KLH production and a Th2-related DTH. The Pb enhancement of DTH in IFNγ-deficient mice is likely due to promotion of type-2 cytokines and enhancement of major histocompatibility complex (MHC) class II expression.
INTRODUCTION
Environmental lead (Pb) contamination can be traced back 5000 years to Ancient Rome (Jarup, 2003). Lead toxicity has been reported to affect the central nervous system, renal function, the cardiovascular system, the immune system, and reproduction (Gidlow, 2004). Although environmental Pb pollution has now been reduced to a remarkable extent, Pb poisoning still occurs (Sen et al., 2002). In addition, Pb exposure may be a lifetime threat to certain Pb-exposed populations during pregnancy, lactation, and post-menopause, as well as during bone injury, in which stored bone Pb can be released back into the blood stream (Gulson et al., 2002, 2003; Nash et al., 2004).
In experimental animals, it has been shown that Pb targets both cell-mediated immunity (CMI) and humoral immunity (HI). It alters CD4+ T-cell–mediated immunity by enhancing Th2 responses and inhibiting Th1 responses (Heo et al., 1996, 1997, 1998). Lead compromises host resistance to bacterial infection (Kim and Lawrence, 2000; Kishikawa et al., 1997), which is due, in part, to the adverse effects of Pb on macrophage functions (Kowolenko et al., 1988; Sengupta and Bishayi, 2002; Tian and Lawrence, 1996; Villanueva et al., 1997). Lead also modifies HI by enhancing T-cell dependent (Lawrence, 1981; Warner and Lawrence, 1986) and -independent (McCabe and Lawrence, 1990) B cell proliferation in vitro, promoting antigen-specific IgG1 production in vivo (Heo et al., 1997), and increasing murine B cell surface expression of MHC class II molecules (McCabe and Lawrence, 1990, 1991).
Production of interferon-gamma (IFNγ) is often used as an index for Th1 responses. Lead exposure has been demonstrated to inhibit IFNγ production in vivo, in vitro, and ex vivo, and to skew immune responses toward Th2-related activities (Heo et al., 1996, 1997, 1998). A Th1 and Th2 imbalance has been implicated in the development of autoimmune diseases, allergic diseases, susceptibility to infectious diseases, progression of AIDS, and spontaneous abortions, as well as in the aging process (Abbas et al., 1996; McCabe, 1994).
In this study, we evaluated Pb effects on cell-mediated immunity (CMI) and humoral immunity (HI) in the absence of IFNγ in vivo. Delayed-type hypersensitivity (DTH) and contact hypersensitivity (CHS) were employed as measures of CMI, while antigen-specific antibody production was used to monitor HI. Our results show that in vivo Pb exposure has differing effects on CMI and on HI. With IFNγ-deficient (IFNγ−/−) mice, Pb exposure skewed CMI toward Th2 responses while lessening the predominance of type-2 HI responses.
MATERIALS AND METHODS
Mice.
Female and male wild-type (WT) and IFN (interferon)−/− BALB/c mice were obtained from the Wadsworth Center animal production unit. Mice were housed in sterile laminar-flow cages in a specified pathogen-free facility of the Wadsworth Center and were maintained on mouse chow and acidified water ad libitum. Mice were 2- to 4-months old at the beginning of the experiments. All of the studies were Institutional Animal Care and Use Committee (IACUC) approved.
Reagents.
Stock solution of 10 mM PbCl2 (Fisher Scientific, Pittsburgh, PA) was prepared in physiological saline (Baxter, Deerfield, IL), and a stock solution of keyhole limpet hemocyanin (KLH) was purchased from Calbiochem (San Diego, CA). All stock solutions were diluted with sterile saline and were confirmed to be endotoxin-free by the use of Limulus Amebocyte Lysate QCL-1000 (BioWhittaker, Walkersville, MD) prior to addition to culture or injection into mice. TiterMax Gold Adjuvant was purchased from Sigma (St. Louis, MO). Culture medium was Roswell Park Memorial Institute (RPMI) 1640 supplemented with 1 mM nonessential amino acids, 1 mM sodium pyruvate, 1% sodium bicarbonate from BioWhittaker (Walkersville, MD), 2 mM glutamine (Sigma), 50 μM β-mercaptoethanol (βME; Fluka, Ronkonkoma, NY), 25 μg/ml gentamicin (Sigma), 1% penicillin-streptomycin-neomycin mixture (Gibco, Grand Island, NY), and 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT). Sterile 1× Dulbecco's phosphate-buffered saline (DPBS) used for cell preparation was purchased from Sigma. Red blood cell lysing buffer was made of 0.017 M Tris and 0.75% NHCl4, pH 7.4.
Pb administration.
Experimental mice were injected with 50 μg PbCl2 in 100 μl saline intraperitoneally (ip) three times per week for 3 weeks, beginning from day 1. Control mice received 100 μl saline on the same schedule. At the end of the third week, Pb level was 65.2 ± 7.6 μg/dl in KO mice; 59.9 ± 10.9 μg/dl in WT mice. Saline-treated controls were all less than 1 μg/dl. The dosage, dosing protocol, and route of administration of Pb followed that used for previous in vivo Pb-skewing the Th1:Th2 responses (Heo et al., 1996, 1997) and that used for mercury induction of autoimmunity (Enestrom and Hultman, 1984; Ochel et al., 1991; Vliet et al., 1993). Preliminary data suggested that this treatment arrangement induced a significant production of IgG2a anti-KLH in Pb-exposed KO mice.
Immunization.
In one experimental group, mice were immunized with KLH (100 μg in 100 μl saline) at day 7 and day 14 during the 3-week course of saline or Pb injections. For the analysis of KLH-specific DTH only, another group of mice were immunized with 100 μg KLH plus TiterMax Gold adjuvant at day 1 and 100 μg KLH in 100 μl saline at day 7; all other immunizations were performed without adjuvant.
Serum preparation.
Peripheral blood was obtained by retro-orbital phlebotomy into 1.7-ml Eppendorf tubes. After it was allowed to clot overnight at 4°C, serum was collected after centrifugation.
Splenocyte preparation.
Mice were euthanized with an overdose of CO2. After 70% ethanol spray, the left flank was opened and the spleen (SPL) removed. The SPL was harvested into a sterile Petri dish containing 2 ml of DPBS without calcium and magnesium (Sigma). Then, it was transferred into another Petri dish containing the same amount of DPBS, inside a biosafety hood. The SPL was homogenized between two frosted microscope slides (Erie Scientific). The cell suspension was transferred into a 15-ml polypropylene tube by use of a Pasteur capillary pipet. After the cell suspension was left for 2–3 min at room temperature to allow separation of the cell debris, the single cell suspension in the supernatant was transferred to a new tube. A red blood cell lysing buffer (0.017 M Tris, 0.75% NH4Cl, pH 7.4; 5 ml) was applied to the pellet after centrifugation at 200 × g for 10 min. After lysis (5 min at room temperature), the cell suspension was centrifuged again, and the cell pellet was resuspended in 10 ml of DPBS; 20 μl of this cell suspension was taken for measurement of the cell concentration. After centrifugation, the cell pellet was resuspended to the appropriate concentration in culture medium.
ELISA for IgG isotype and IgE.
Immunoglobulin G isotype and IgE were measured by a standard ELISA assay. For measurement of KLH-specific IgG1 and IgG2a, KLH (10 μg/ml) in 0.1 M carbonate pH 9.5 (8.4 g NaHCO3, 3.56 g Na2CO3, in 1 liter) was coated onto Costar ELISA plates overnight at 4°C and then washed. After a 2-h blocking with phosphate buffered saline (PBS) plus 1% bovine serum albumin (BSA; 200 μl/well) at room temperature and then a wash, a serial dilution of the standard (mouse IgG2a anti-KLH; Sigma) or serum was added in duplicate to standard or sample wells, respectively, and incubated overnight at 4°C. Then, biotinylated detection mAbs (biotin anti-mouse IgG1 from BD Science for IgG1 detection or biotin anti-mouse IgG2a from BD Science for IgG2a detection) were applied following six washings. After 1 h of incubation at room temperature, the plates were washed, and avidin-peroxidase (PharMingen) was added. After a further 30-min incubation and washing, the substrate (3,3,5,5-tetramethyl-benzidine; Sigma) was applied. The reaction was stopped with the addition of 1 M H2SO4. The plates were read using an ELISA reader (EL310, Bio-Tek, Burlington, VT) at 450 nm.
For measurement of KLH-specific IgE, 2 μg/ml purified anti-mouse IgE (PharMingen) was coated onto Costar ELISA plates overnight at 4°C. After washing and a 2-h blocking with PBS plus 1% BSA (200 μl/well) at room temperature, a serial dilution of the standard (mouse IgE, PharMingen) and serum was added and incubated overnight at 4°C. After the sample was washed, KLH (10 μg/ml) in 0.1 M carbonate pH 9.5 was added into sample wells only. After 2-h incubation at room temperature, mouse IgG2a anti-KLH, followed by Biotin anti-mouse IgG2a, avidin-peroxidase was applied. A 6-time washing was used among the steps. For the standard development, 2 μg/ml biotin anti-mouse IgE (PharMingen) and avidin-peroxidase were used. The same method was utilized for color development and for reading of the plates.
For measurement of total IgE, OptEIA Mouse IgE Set (PharMingen) was employed. The protocol provided by the manufacturer was used. The plates were read in the same way described above.
Flow-cytometric analysis.
Single-cell suspensions of popliteal lymph node (PLN) cells were prepared and analyzed by four-color flow cytometry (FACSCaliber, Becton Dickinson & Co.). Cells (106) were suspended in 100 μl PBS containing 0.1% azide and 1 μg of various directly conjugated mAbs. Allophycocyanin (APC) anti-CD19, Cy5.5 anti-CD11b, phycoerythrin (PE) anti-I-Ad, and fluorescein isothiocyanate (FITC) anti-ICAM-1 were grouped together, whereas APC anti-CD8, PerCP anti-CD4, PE anti-CD86, and FITC anti-Pan-NK were grouped together. After 30 min of incubation on ice, cells were washed and analyzed on the flow cytometer by gating for lymphocytes with the appropriate settings for forward- and side-light scatter.
Cell culture.
After DTH, spleen (SPL) and peripheral lymph node (PLN) cells were harvested. Single-cell suspensions of SPL or PLN cells were cultured in complete Roswell Park Memorial Institute (RPMI) medium at 37°C in a low O2 (5% O2) incubator. After 3 days (for PLN only) or 5 days with 50 μg/ml KLH (for SPL only), culture supernatant (SN) was collected and stored at −20°C for cytokine analysis.
Cell proliferation.
Culture cells were pulsed with 0.5 μCi/well 3H-thymidine (Dupont-NEN, Wilmington, DE) during the last 6 h of culture. Cells were harvested by a Basic 96 Harvester (Skatron Instruments, Lier, Norway). The phosphostimulated luminesence image was read by a BAS 2000 Fujix reader and analyzed by the TINA 2.0 program.
Bio-Plex cytokine assays.
Cytokines were detected from culture supernatant samples by the Upstate cytokine assay kit (Upstate Biologicals, Lake Placid, NY, USA). The assay protocol was provided by manufacturer. The assay was run on a Luminex 100 instrument. The results were analyzed by using Sigma fitting curves and Excel spread sheet.
DTH assay.
Delayed-type hypersensitivity assays were performed 21 days after the last immunization with KLH. After measurement of the thickness of the left and right footpad of each mouse, KLH (100 μg/25 μl saline) was subcutaneously injected into the right footpad and saline only was injected into the left footpad. The DTH responses were measured 24 h later with a Spi dial thickness gauge. The responses were defined as the difference between the right and left footpad swellings.
CHS assay.
The CHS assay was performed at day 28 after 3-week saline or Pb injection. First, 20 μl of 0.5% DNFB in 4:1 acetone/olive oil was painted onto the shaved abdomen. After 5 days, 20 μl of 0.2% DNFB in 4:1 acetone/olive oil was challenged on one ear and the solvent control was painted onto the other ear. The CHS response was measured 24 h later with a Spi dial thickness gauge (Swiss Precision Instruments). The responses were defined as the difference between the right and left ear swelling.
RNA Isolation from popliteal lymph node cells.
The cell pellet was resuspended in 50 μl of medium before addition of the RLT/βME lysing solution from Qiagen, Inc. (Valencia, CA). After cell lysis using the RLT/βME solution, lysates were homogenized with the Shredder Spin Columns provide by Qiagen. RNA was isolated using the Qiagen RNA capture mini-columns according to the manufacturer's protocol for isolation of RNA from cultured cells. Isolated RNA, in 60 μl of RNase-free water, was quantitated by measurement of the absorbance at 260 nm, and RNA purity was determined by calculating the 260/280 absorbance ratio using a Beckman DU 640 Spectrophotometer. Total RNA yields ranged between 11 and 14 μg per culture condition and per time period.
Quantitative real-time RT-PCR.
A two-step process was employed for mRNA quantification. First, cDNA was prepared from 1μg of total RNA using a first-strand cDNA synthesis kit from Roche Applied Science (Indianapolis, IN). Following the synthesis reaction, an aliquot of 200 μl PCR-grade water was added to each tube. The second step involved separate amplification for each cytokine gene sequence using primer kits from Search-LC (Heidelberg, Germany). Amplifications were carried out in duplicate in a Roche LightCycler instrument under conditions specified by Search-LC. Standard curves were generated for each run using standards of known copy number supplied by Search-LC. Quantification was performed for the following cytokine mRNAs: IL-4, IL-5, IL-10, IL-12p40, IL-15, and IL-18. Quantitation results were recorded as copy number per μl (CN), and all cytokine results were normalized to the CN obtained for GAPDH from the same cDNA synthesis sample.
Statistical analysis.
Statistical analysis was performed by SigmaStat (Jandeel Scientific, San Rafael, CA) t-test, or Mann-Whitney rank sum test; p ◂ 0.05 was considered significant.
RESULTS
In Vivo Pb Exposure Induced a Th1-Skewing of HI in the IFNγ−/−, but Not the WT, BALB/c Mice
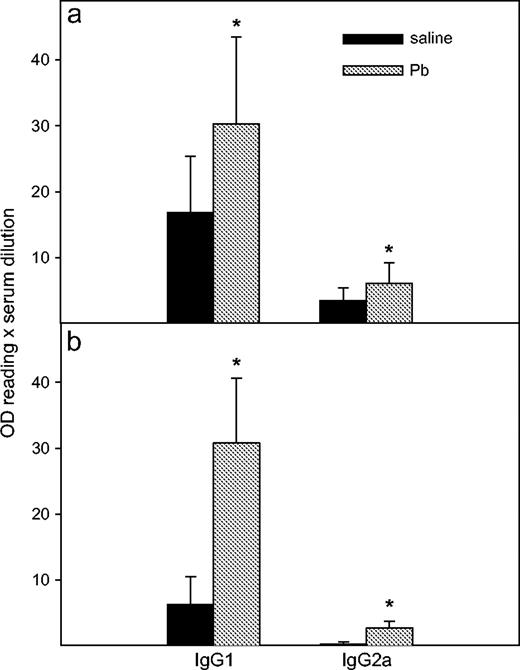
Sera from the Pb- and saline-treated mice were tested for IgG1and IgG2a anti-KLH production. IgG1 (p < 0.0001) and IgG2a (p < 0.0001) anti-KLH production was significantly impaired in control IFNγ−/− mice, compared with control WT mice. In BALB/c WT mice (Fig. 1a), Pb exposure enhanced both IgG1 (30.3 ± 13.2, p = 0.002) and IgG2a (6.1 ± 3.1; p = 0.01) anti-KLH production, compared with control mice (16.8 ± 8.6 and 3.5 ± 1.9, respectively). The increases of IgG1 and IgG2a anti-KLH were about 1.8-fold and 1.6-fold, respectively, in Pb-exposed WT mice, indicating equivalent enhancement. Lead exposure also significantly increased IgG1 (30.8 ± 9.8; control = 6.3 ± 4.2; p < 0.0001) and IgG2a (2.7 ± 1.1; control = 0.3 ± 0.3; p < 0.001) anti-KLH production in the IFNγ−/− mice (Fig. 1b). However, IgG1 and IgG2a anti-KLH levels were increased fivefold and about ninefold, respectively, suggesting that a greater Th1-skewing occurred in Pb-treated IFNγ−/−mice. The enhanced type-1 skewing in the IFNγ−/− mice is also apparent with the IgG1:IgG2a ratios (WT: 5.3 ± 2.0 without Pb and 6.1 ± 3.7 with Pb versus IFNγ−/−: 22.2 ± 8.9 without Pb and 14.0 ± 7.9 with Pb).
Effects of Pb treatment on anti-KLH IgG isotype production. WT (a) and IFNγ−/− (b) BALB/c mice were injected ip with PbCl2 or saline and immunized, after which sera were collected, as described (Materials and Methods). The levels of serum IgG isotypes were determined by ELISA. Results were obtained from three separate experiments with n = 16 for each WT group and n = 15 for each IFNγ−/− group. Data are presented as mean OD values ± SD. Statistically significant (p < 0.05) differences from saline values are designated with an asterisk.
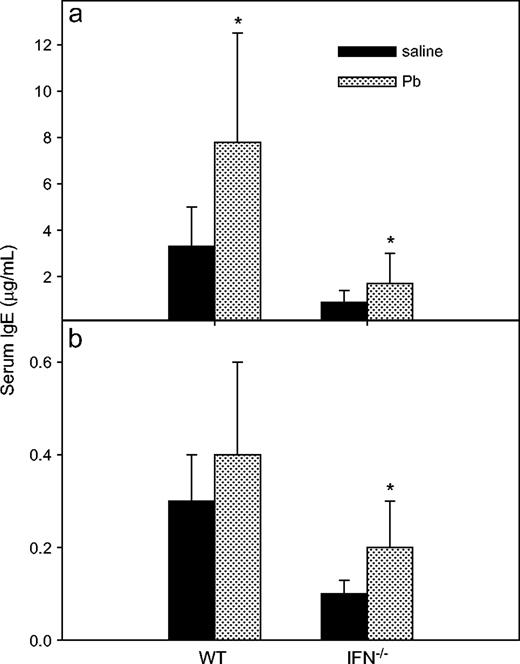
In vivo Pb exposure enhanced total IgE in both WT and IFNγ−/− BALB/c mice, but it enhanced anti-KLH IgE production only in the IFNγ−/− mice. Overall, the total serum IgE levels were greater (p < 0.0001) in WT mice than in the IFNγ−/− mice (Fig. 2). Pb exposure did not significantly affect anti-KLH IgE production after the secondary immunization with KLH in WT mice (p = 0.07) (Fig. 2), but Pb significantly enhanced the IgE anti-KLH levels in the IFNγ−/− mice (p = 0.003). Total IgE production was significantly enhanced by Pb in both types of mice (p = 0.004 in WT mice; p = 0.01 in KO mice).
Effects of Pb treatment on serum total IgE and IgE anti-KLH levels. The same sera collected from WT and IFNγ KO BALB/c mice for anti-KLH IgG (Fig. 1) were used for assessment of total serum IgE (a) or IgE anti-KLH (b) by ELISA. Results were obtained from three separate experiments with n =14 and n = 16 for the control and Pb-treated WT groups, respectively; each IFNγ−/− group had n = 15. Data are presented as mean μg/ml ± SD of three separate experiments with use of standard IgE serum; an asterisk designates statistically significant (p < 0.05) differences from saline values.
In Vivo Pb Exposure Induced a Strong KLH-Specific DTH Response in the IFNγ−/− BALB/c Mice
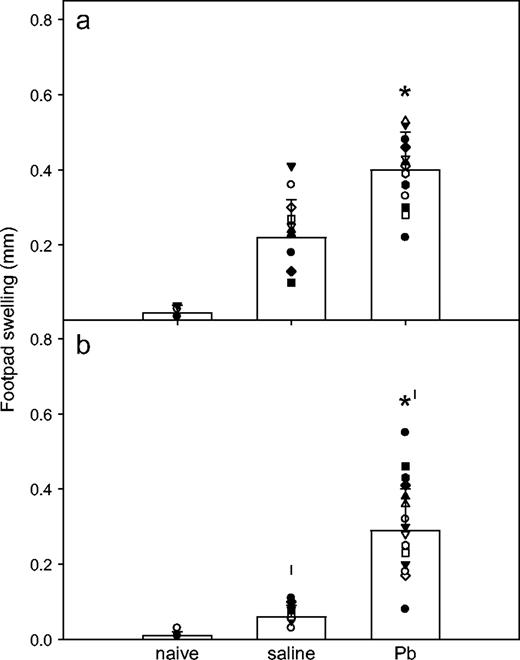
To evaluate the ability of Pb to modify CMI, at day 35, we challenged all mice in the footpad with 100 μg KLH (25 μl). Unlike previous reports (Faith et al., 1979; McCabe et al., 1999; Miller et al., 1998; Muller et al., 1977), Pb exposure significantly (p < 0.001) enhanced DTH responses in WT mice (0.22 ± 0.1 mm for controls; 0.4 ± 0.1 mm for Pb-treated mice; Fig. 3a). Not surprisingly, low DTH responses (p < 0.0001, compared with the WT mice) were detected for the IFNγ−/− mice (0.06 ± 0.03 mm; Fig. 3b). However, most interestingly, the IFNγ−/− mice developed significantly (p < 0.001) enhanced DTH responses when treated with Pb (0.29 ± 0.11 mm). The Pb may act as an adjuvant, in that when the primary immunization with KLH was given with TiterMax Gold, the WT and the IFNγ−/− DTH responses were both significantly enhanced (0.55 ± 0.05 mm for WT and 0.26 ± 0.15 mm for IFNγ−/−).
Effects of Pb treatment on DTH responses. After previous assessment for serum IgG and IgE levels, as described (Fig. 1), the WT (a) and IFNγ−/− (b) BALB/c mice received a footpad challenge (100 μg KLH in 25 μl saline) at day 35. The DTH response was measured 24 h later. Naïve WT (n = 10) and IFNγ−/− (n = 11) mice represent the negative controls from three separate experiments. Results for Pb-exposed (n = 24) and control (n = 25) WT mice and Pb-exposed (n = 25) and control (n = 27) IFNγ−/− mice are shown as mean ± SD as bar graphs. Symbols within each bar graph represent individual mouse values from six separate experiments; * and † designate statistically significant (p < 0.05) differences from saline values and WT values, respectively.
In Vivo Pb Exposure Increased Th2 Cytokine Production in PLN or SPL Culture Supernatants from WT or IFNγ−/− BALB/c Mice
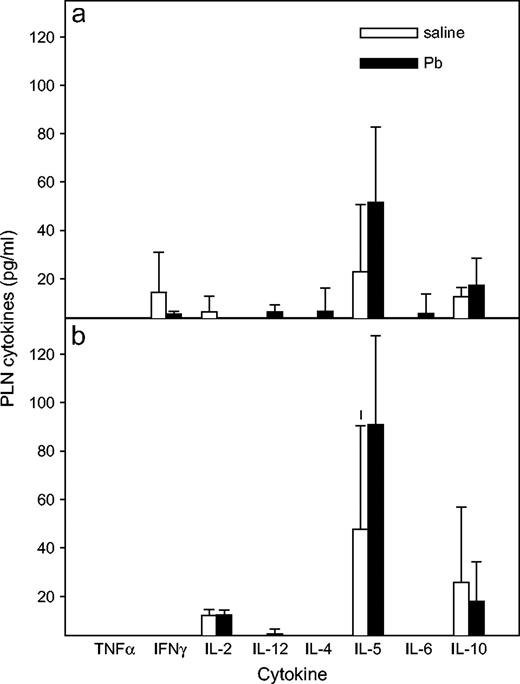
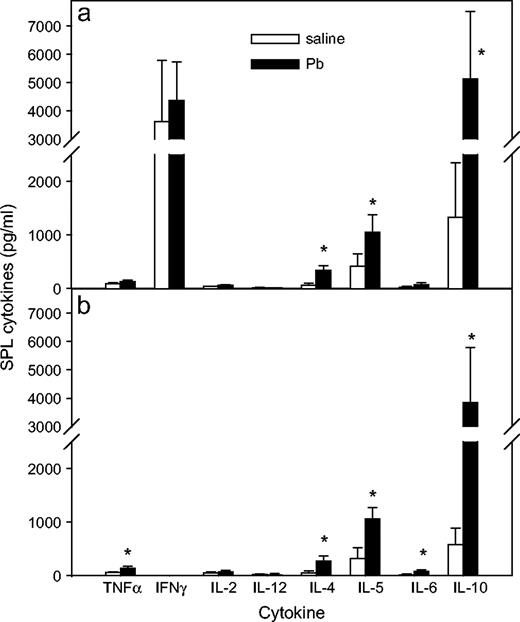
To aid assessment of the types of immune factors associated with the KLH-specific DTH response (PLN reactivity) versus the more systemic response (SPL reactivity), all mice were euthanized, and PLNs and SPLs were harvested after DTH measurement. The footpad draining PLN cells were cultured without any additional KLH in vitro for 3 days in order to more closely approximate the response to the in vivo stimulation, whereas SPL cells were stimulated again in vitro with KLH (50 μg/ml) for 5 days, and culture supernatants (SN) were tested for various cytokines. Overall, tumor necrosis factor-alpha (TNFα) and IFNγ levels were significantly lower in PLN (p = 0.0485, p = 0.038, respectively) and SPL (p = 0.0487, p < 0.0001, respectively) culture SN from control IFNγ−/− mice compared to the WT mice. No significant differences of IL-2, IL-12, IL-4, IL-5, IL-6, and IL-10 were observed in culture SN from either strain of control mice. As shown in Figure 4, more Th2 cytokines were produced by the cells from WT and IFNγ−/− mice in the 3-day PLN culture SN. The cytokine amounts produced by PLNs from Pb-exposed WT mice (Fig. 4a) were not significantly different from the control WT mice, although the Th2 cytokines (IL-4, IL-5, and IL-10) were increased more than the Th1 cytokines (IL-2 and IFNγ). A similar pattern was observed in PLN culture SN from the IFNγ−/− mice (Fig. 4b). Lead significantly enhanced SPL production of IL-4, IL-5, and IL-10 (Th2-type cytokines) in both WT and IFNγ−/− mice, whereas Pb had no significant affect on the production of IFNγ, IL-2, or IL-12 (Th1-type cytokines) in either mouse type. In the IFNγ−/− mice (Fig. 5b), Pb also significantly enhanced SPL production of TNFα and IL-6. The IFNγ level was at the limit of the assay sensitivity, indicating that the IFNγ−/− mice are not leaky.
Effects of Pb treatment on cytokine production by PLN cells. At day 36, after KLH DTH measurement, PLN cells were harvested from WT (a) and IFNγ−/− (b) BALB/c mice and cultured at 2 × 105 cells /200 μl per well in 96-well plates. After a 3-day incubation period, culture SN was collected and assayed for multi-cytokine production. Results for Pb-exposed (n = 5) and control (n = 4) WT mice and Pb-exposed (n = 5) and control (n = 4) IFNγ−/− mice are shown as mean ± SD from one experiment; * designates p < 0.05.
Effects of Pb treatment on cytokine production by SPL cells. At day 36, after KLH DTH measurement, SPL cells were harvested from WT (a) and IFNγ−/− (b) BALB/c mice and cultured at 2 × 105 cells /200 μl per well in 96-well plate with KLH for 5 days, at which time supernatants were collected and assayed for multi-cytokine production. Results for Pb-exposed (n = 5) and control (n = 4) WT mice and Pb-exposed (n = 6) and control (n = 8) IFNγ−/− mice are shown as mean ± SD from two separate experiments; * designates p < 0.05.
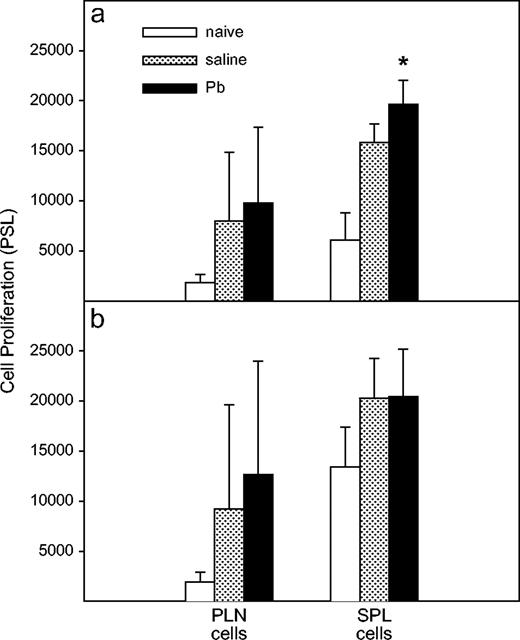
In Vivo Pb Exposure Increased PLN and SPL Cell Proliferation in WT Mice, but Not in IFNγ−/− Mice
To further investigate T-cell function, we examined in vitro proliferative ability of 3-day cultured PLN cells or 5-day cultured SPL cells. In general, the proliferation of naive SPL cells from IFNγ−/− mice was greater (p = 0.0062) than that of WT cells. Lead exposure significantly (p = 0.04) enhanced SPL cell proliferation in WT mice (Fig. 6a). The proliferation was not affected by Pb exposure of IFNγ−/− mice (Fig. 6b).
Effects of Pb treatment on in vitro proliferation of PLN or SPL cells. After collection of 100 μl of supernatant per well for the cytokine production assay, the WT (a) and IFNγ−/− (b) cells were pulsed with 0.5 μCi /100 μl of 3H-thymidine per well for 6 h and then harvested. The proliferation was determined by Fujix BAS 2000 Image Scanner. The cells of naïve WT (n = 7) and IFNγ−/− (n = 7) mice (two experiments) represent the baseline proliferation. Results for PLN cells from Pb-exposed (n = 15) and control (n = 15) WT mice and Pb-exposed (n =16) and control (n = 17) IFNγ−/− mice are shown as mean ± SD from four separate experiments. Results for SPL cells from Pb-exposed (n = 5) and control (n = 4) WT mice and Pb-exposed (n = 6) and control (n = 8) IFNγ−/− mice are shown as mean ± SD from one experiment. The values represent the mean ± SD; * designates p < 0.05.
In Vivo Pb Exposure Increased Expression of MHCII on CD11b+ Cells of the IFNγ−/− PLN Cells
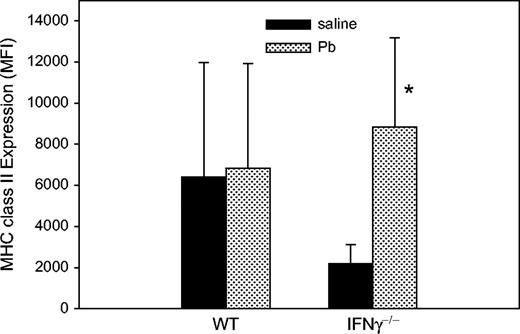
After measurement of the DTH response to KLH, we stained fresh PLN cells with various markers to assess phenotypic changes after Pb treatment. Expressions of surface molecules were detected by flow cytometry. Expression of CD4, CD8, B, NK, ICAM, and B7.2, as well as CD11b, was not significantly changed by Pb exposure in either type of mice (data not shown). The impaired MHC II expression (MFI was 2188 ± 938) on CD11b+ cells from the IFNγ−/− mice is consistent with findings of a previous report (Dalton et al., 1993). Surprisingly, Pb exposure enhanced (p = 0.04) MHC II expression (MFI was 8849 ± 4331) on CD11b+ cells from the IFNγ−/− mice (Fig. 7).
Effects of Pb treatment on the surface expression of MHC class II on CD11b+ PLN cells. At day 36, after the KLH DTH measurement, PLN cells from WT (a) and IFNγ−/− (b) BALB/c mice were examined for MHC II expression. MFI was 6397 ± 5579 for control WT mice (n = 5) and 6822 ± 5094 for Pb-exposed WT mice (n = 6). MFI was increased fourfold on Pb-exposed IFNγ−/− mice (n = 4) in comparison to the control IFNγ−/− mice (n = 5). Data are presented as mean ± SD of one experiment; * designates p < 0.05.
Cytokine mRNA Measurement of PLNs from WT and IFNγ−/− BALB/c Mice
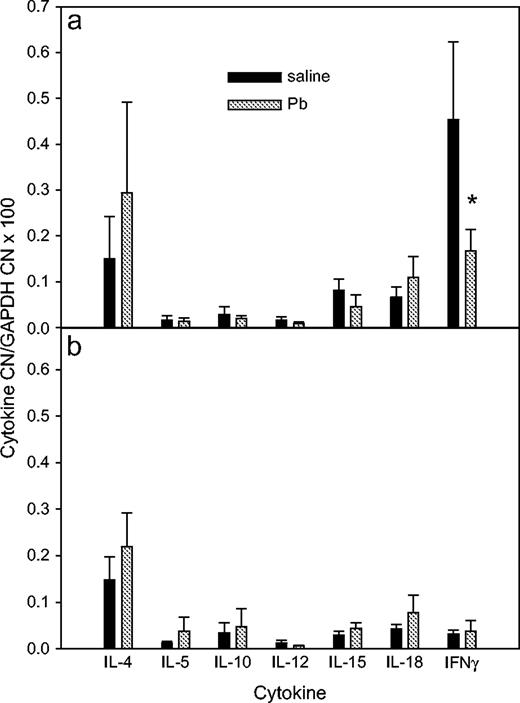
There were no significant mRNA differences for any cytokine except IFNγ, between the WT and KO mice (Fig. 8). Additionally, Pb did not induce any significant changes in any measured cytokine mRNA levels in either WT or KO mice. Interestingly, the IL-4 to IFNγ mRNA ratio was higher in the PLN preparation from the WT mice after Pb exposure; however, none of the cytokine mRNA levels can account for the increased DTH of the Pb-treated IFNγ−/− mice.
Effects of Pb treatment on cytokine mRNA expression by PLNs. At day 36, after the KLH DTH measurement, RNA was isolated from WT (a) and IFNγ−/− (b) BALB/c PLNs.
In Vivo Pb Exposure Did Not Affect the DNFB CHS Response in BALB/c WT and IFNγ−/− Mice
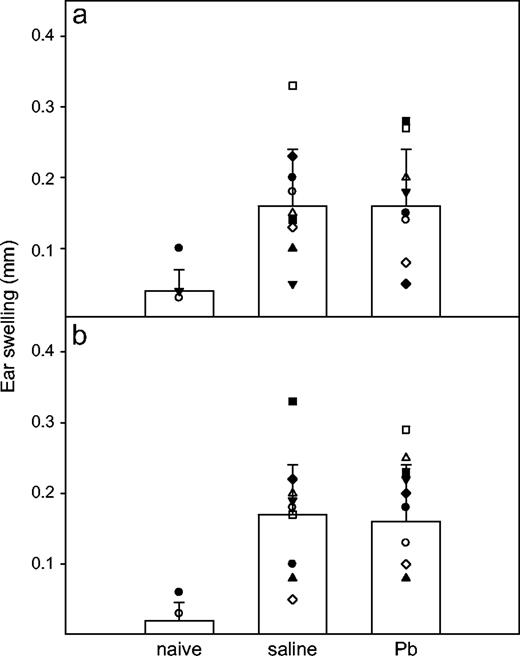
For assessment of Pb effects on the CHS response, a response that is thought to be mainly due to CD8+ T cells (Anderson et al., 1995; Bour et al., 1995; Gocinski and Tigelaar, 1990; Xu et al., 1996), the mice received 3-week Pb exposure as before. At day 28, the mice were sensitized with DNFB on the shaved left abdomen, and 5 days later, they were challenged with DNFB on one ear. In general, the IFNγ−/− mice had the same ability to develop DNFS CHS response as the WT mice. Figure 9 shows that Pb exposure did not affect the DNFB CHS responses in either the WT (p = 0.86) or IFNγ−/− (p = 0.85) mice, indicating that Pb has less affect on CD8+ T cells. In addition, it appears that IFNγ is not required for CHS to occur, in that the WT and KO mice had equivalent responses.
Effects of Pb treatment on DNFB CHS responses. Groups of WT (a) and IFNγ−/− (b) BALB/c mice received the same Pb or saline treatment, as described, but they had no KLH immunization. DNFB sensitization and elicitation were performed as described in Materials and Methods. CHS of naïve WT (n = 5) and KO (n = 5) mice served for baseline ear swelling. Results for Pb-exposed (n = 12) and control (n = 12) WT mice and Pb-exposed (n = 15) and control (n = 13) IFNγ−/− mice are shown. Symbols within each bar graph represent individual mouse values from two separate experiments. Data are presented as mean ± SD.
DISCUSSION
The present study was designed to evaluate in vivo Pb effects on CMI and HI in the absence of IFNγ. We have elucidated a unique phenomenon induced by Pb in IFNγ KO mice, namely, enhancement of type-1–related HI and type-2–related CMI responses in IFNγ KO mice. The mixed Th1/Th2 response has been reported in a vaccine study of BALB/c mice (Mazumdar et al., 2004). Previously, based on findings of enhanced IL-4 production and inhibited IFNγ production, Pb was reported to promote Th2 skewing in vivo in WT BALB/c mice (Heo et al., 1996). As expected, the absence of IFNγ significantly lowered the production of IgG2a antibodies to KLH. Surprisingly, Pb promoted IgG2a anti-KLH levels, even though Pb preferentially induces Th2 cytokines.
Usually, the Th2 cytokine IL-4 induces the secretion of IgG1 and IgE (Bossie et al., 1987; Vitetta et al., 1984), whereas the Th1 cytokine IFNγ directs the secretion of IgG2a (Snapper and Paul, 1987; Stevens et al., 1988). Recently, an IFNγ–independent, IL-27–dependent, STAT1-dependent pathway has been clarified (Chen et al., 2000; Takeda et al., 2003; Yoshida et al., 2001; Yoshimoto et al., 2004); this pathway is involved in early initiation of Th1 responses and B-cell switching to IgG2a. Lead may alter an IFNγ-independent pathway, such as the IL-27 pathway or, perhaps, other pathways, to enhance IgG2a levels because IFNγ production was not significantly affected in Pb-exposed WT mice and because the IFNγ−/− mice have no IFNγ production. IgG1 and IgE levels are associated with IL-4 cytokine production. Therefore, not surprisingly, IgG1 and IgE levels were increased in both types of mice because Pb exposure enhanced IL-4 production. Based on the IgG1:IgG2a ratio, the present results suggest that Pb exposure induced Th2 skewing in WT mice. This is consistent with previous reports (Heo et al., 1996, 1997, 1998). However, Pb lowered the IgG1:IgG2a ratio in IFNγ KO mice, which suggests that Pb lessens type-2 HI skewing in the absence of IFNγ.
Although our current results confirm, based on the enhancement of type-2 cytokines, that Pb induces a Th2 skewing, we also show enhancement of DTH, which is usually considered a type-1 immune response (in that Th1 CD4+ T cells mediate the classic DTH response, the tuberculin reaction). Unlike Th1 DTH responses, some other DTH responses associated with large basophil infiltrates are mediated by Th2 cells (Askenase, 2000; Dvorak et al., 1970, 1971). This type of DTH response is also called cutaneous basophil hypersensitivity (CBH). B cells and antibodies (IgG1 and IgE) are also involved in CBH (Askenase, 1976; Stashenko et al., 1977). Lead exposure mounted a strong DTH response, which is likely a Th2 DTH response. Recent studies have implicated IL-5 in drug-induced skin hypersensitivities (Engler et al., 2004; Sachs et al., 2002), which further suggests involvement of type-2 immunity. Because a Th2-induced DTH has been suggested to be associated with asthma (Askenase, 2000), Pb exposure may be linked to asthma, which has been suggested (Lutz et al., 1999; Snyder et al., 2000).
It has also been suggested that IFNγ is required for both Th1 (Fong and Mosmann, 1989) and Th2 (Akahira-Azuma et al., 2004) DTH development. As expected, we found a minimal DTH response in IFNγ KO mice. Surprisingly, Pb exposure caused a strong DTH response in the absence of IFNγ, suggesting that Pb exposure can induce the DTH response through an IFNγ independent pathway. Thus, the induction of this DTH response in the absence of IFNγ is deemed a Th2 DTH response. This finding disagrees with previous reports that Pb exposure inhibits a DTH response (Faith et al., 1979; McCabe et al., 1999; Miller et al., 1998; Muller et al., 1977). Differences between our study and previous studies include the route and dosage of Pb and of antigen, the antigen itself, and the type of experimental animals employed. The route of antigen administration has significant effects on the DTH response (McCabe et al., 1999; Stertman et al., 2004). None of the prior studies used the route of antigen administration that we used in the current study. This could be one of the reasons for different results. Another possibility is their use of adjuvant. In the present study, the IFNγ−/− mice that were immunized with antigen alone had a low DTH response, whereas those immunized with antigen plus a single dose of the TiterMax Gold adjuvant (or with Pb dosing) mounted strong DTH responses. It is reasonable to predict that the Pb effects on DTH could be concealed by the adjuvant, if the adjuvant were applied with an antigen. On the other hand, our result may indicate that Pb preferentially aids antigen-presenting cells promoting HI. The presence or the specific type of adjuvant is not only inducing different degrees of DTH response, it is also leading to a different type (Th1 or Th2) of DTH response (Cribbs et al., 2003).
There is evidence that NK, NKT, or T cells can be responsible for a DTH response (Askenase et al., 2004). Because IL-4, IL-5, and IL-10 are the predominate cytokines enhanced by Pb, it seems that NKT cells, which can be skewed toward IL-4 or IFNγ producers (Matsumoto et al., 2004), could be a major participant. Although a DTH response is usually utilized to evaluate CMI, it is not always a good indicator of CMI potential. For example, C57BL/6 IFNγ−/− mice that developed a Th2 DTH response failed to control chlamydial infection (Wang et al., 1999). In any case, a DTH response does not necessarily confirm CMI. In addition to Pb promoting a strong DTH in the absence of IFNγ, Pb enhanced MHC class II expression. IFNγ−/− mice show impaired expression of macrophage MHC II antigen (Dalton et al., 1993); thus, Pb-enhanced expression of MHC class II on CD11b+ cells of the IFNγ−/− mice is another paradox, as this also could be interpreted as enhancement of CMI. Although the mechanism for the Pb enhancement of the DTH response in the absence of IFNγ is not clear, the increase in type-2 cytokines and the previous reports of Pb enhancement of B-cell activation (McCabe and Lawrence, 1990, 1991), suggests that B-cell involvement in T-cell activation may be implicated. Lead may promote more cognate B–T-cell interactions than macrophage–T-cell interactions, leading to enhancement of HI and type-2 DTH responses as opposed to CMI and type-1 DTH responses. Alternatively, Pb may alter dendritic cell (DC) interactions with Th1 versus Th2 cells in a fashion similar to the differential responses obtained with antigen-pulsed DC injected subcutaneously versus intravenuously (Kuribayashi et al., 1997). Like the results described with Pb, subcutaneous DC induced Th2-mediated DTH, indicating deviant Th1 and Th2 responses.
We also investigated CHS, so as to evaluate Pb effects on another response usually considered to be CMI. It is generally believed that a CHS response, unlike a DTH response, is mediated by CD8+ T cells (Anderson et al., 1995; Bour et al., 1995; Gocinski and Tigelaar, 1990; Xu et al., 1996). It has been proposed that CD4+ T cells are required for optimum induction of CHS (Fujisawa et al., 1996; Kondo et al., 1996). The data in the current study indicate that Pb has less effect on CD8+ T cell–mediated immune response. Moreover, it appears that IFNγ is not required for the CHS to occur.
In conclusion, Pb induces a unique Th1/Th2 mixed immune response in BALB/c IFNγ−/− mice. Although the mechanism behind this phenomenon needs to be further investigated, we have shown that it is not due to increased expression of IL-12, IL-15, or IL-18 (cytokines known to enhance CMI); whereas IL-4 and IL-5 are implicated. Paradoxically, Pb inhibits IFNγ production (Heo et al., 1996, 1997, 1998) and CMI (Kim and Lawrence, 2000; Kishikawa et al., 1997) in WT BALB/c mice, whereas in IFNγ−/− BALB/c mice, Pb enhances immune functions usually thought to be associated with IFNγ.
The authors thank the staff of the Immunology Core of Wadsworth Center for their assistance with the flow cytometry and phosphorimaging analysis. The research was supported in part by grant ES11135 from the National Institute of Environmental Health Services (NIEHS).
References
Abbas, A. K., Murphy, K. M., and Sher, A. (
Akahira-Azuma, M., Szczepanik, M., Tsuji, R. F., Campos, R. A., Itakura, A., Mobini, N., McNiff, J., Kawikova, I., Lu, B., Gerard, C., et al. (
Anderson, C., Hehr, A., Robbins, R., Hasan, R., Athar, M., Mukhtar, H., and Elmets, C. A. (
Askenase, P. W. (
Askenase, P. W. (
Askenase, P. W., Szczepanik, M., Itakura, A., Kiener, C., and Campos, R. A. (
Bossie, A., Brooks, K. H., Krammer, P. H., and Vitetta, E. S. (
Bour, H., Peyron, E., Gaucherand, M., Garrigue, J. L., Desvignes, C., Kaiserlian, D., Revillard, J. P., and Nicolas, J. F. (
Chen, Q., Ghilardi, N., Wang, H., Baker, T., Xie, M. H., Gurney, A., Grewal, I. S., and de Sauvage, F. J. (
Cribbs, D. H., Ghochikyan, A., Vasilevko, V., Tran, M., Petrushina, I., Sadzikava, N., Babikyan, D., Kesslak, P., Kieber-Emmons, T., Cotman, C. W., et al. (
Dalton, D. K., Pitts-Meek, S., Keshav, S., Figari, I. S., Bradley, A., and Stewart, T. A. (
Dvorak, H. F., Dvorak, A. M., Simpson, B. A., Richerson, H. B., Leskowitz, S., and Karnovsky, M. J. (
Dvorak, H. F., Simpson, B. A., Bast, R. C. J., and Leskowitz, S. (
Enestrom, S., and Hultman, P. (
Engler, O. B., Strasser, I., Naisbitt, D. J., Cerny, A., and Pichler, W. J. (
Faith, R. E., Luster, M. I., and Kimmel, C. A. (
Fong, T. A., and Mosmann, T. R. (
Fujisawa, H., Kondo, S., Wang, B., Shivji, G. M., and Sauder, D. N. (
Gocinski, B. L., and Tigelaar, R. E. (
Gulson, B., Mizon, K., Smith, H., Eisman, J., Palmer, J., Korsch, M., Donnelly, J., and Waite, K. (
Gulson, B. L., Mizon, K. J., Korsch, M. J., Palmer, J. M., and Donnelly, J. B. (
Heo, Y., Lee, W. T., and Lawrence, D. A. (
Heo, Y., Lee, W. T., and Lawrence, D. A. (
Heo, Y., Parsons, P. J., and Lawrence, D. A. (
Kim, D., and Lawrence, D. A. (
Kishikawa, H., Song, R., and Lawrence, D. A. (
Kondo, S., Beissert, S., Wang, B., Fujisawa, H., Kooshesh, F., Stratigos, A., Granstein, R. D., Mak, T. W., and Sauder, D. N. (
Kowolenko, M., Tracy, L., Mudzinski, S., and Lawrence, D. A. (
Kuribayashi, K., Tsukiyama, M., and Takenaka, T. (
Lawrence, D. A. (
Lutz, P. M., Wilson, T. J., Ireland, J., Jones, A. L., Gorman, J. S., Gale, N. L., Johnson, J. C., and Hewett, J. E. (
Matsumoto, G., Kubota, E., Omi, Y., Lee, U., and Penninger, J. M. (
Mazumdar, T., Anam, K., and Ali, N. (
McCabe, M. J. (
McCabe, M. J., and Lawrence, D. A. (
McCabe, M. J., and Lawrence, D. A. (
McCabe, M. J., Singh, K. P., and Reiners, J. J. (
Miller, T. E., Golemboski, K. A., Ha, R. S., Bunn, T., Sanders, F. S., and Dietert, R. R. (
Muller, S., Gillert, K. E., Krause, C., Gross, U., L'Age-Stehr, J., and Diamanstein, T. (
Nash, D., Magder, L. S., Sherwin, R., Rubin, R. J., and Silbergeld, E. K. (
Ochel, M., Vohr, H. W., Pfeiffer, C., and Gleichmann, E. (
Sachs, B., Erdmann, S., Malte Baron, J., Neis, M., al Masaoudi, T., and H. F. M. (
Sen, D., Wolfson, H., and Dilworth, M. (
Sengupta, M., and Bishayi, B. (
Snapper, C. M., and Paul, W. E. (
Snyder, J. E., Filipov, N. M., Parsons, P. J., and Lawrence, D. A. (
Stashenko, P. P., Bhan, A. K., Schlossman, S. F., and McCluskey, R. T. (
Stertman, L., Strindelius, L., and Sjoholm, I. (
Stevens, T. L., Bossie, A., Sanders, V. M., Fernandez-Botran, R., Coffman, R. L., Mosmann, T. R., and Vitetta, E. S. (
Takeda, A., Hamano, S., Yamanaka, A., Hanada, T., Ishibashi, T., Mak, T. W., Yoshimura, A., and Yoshida, H. (
Tian, L., and Lawrence, D. A. (
Villanueva, R., Albaladejo, R., Ortega, P., Astasio, P., Gil, A., Calle, M. E., and Dominguez-Rojas, V. (
Vitetta, E. S., Brooks, K., Chen, Y. W., Isakson, P., Jones, S., Layton, J., Mishra, G. C., Pure, E., Weiss, E., Word, C., et al. (
Vliet, E. V., Uhrberg, M., Stein, C., and Gleichmann, E. (
Wang, S., Fan, Y., Brunham, R. C., and Yang, X. (
Warner, G. L., and Lawrence, D. A. (
Xu, H., DiIulio, N. A., and Fairchild, R. L. (
Yoshida, H., Hamano, S., Senaldi, G., Covey, T., Faggioni, R., Mu, S., Xia, M., Wakeham, A. C., Nishina, H., Potter, J., et al. (




Comments