-
PDF
- Split View
-
Views
-
Cite
Cite
A.J. Todd, S.J. Leslie, M. MacDougall, M.A. Denvir, Clinical features remain important for the diagnosis of infective endocarditis in the modern era, QJM: An International Journal of Medicine, Volume 99, Issue 1, January 2006, Pages 23–31, https://doi.org/10.1093/qjmed/hci150
Close - Share Icon Share
Abstract
Background: Infective endocarditis (IE) can be difficult to diagnose, due to multiple (often non-specific) presenting features.
Aim: To assess the predictive accuracy of classical clinical features and blood investigations readily available at the time of presentation.
Design: Cross-sectional analysis.
Methods: We studied 29 IE cases and 79 controls (clinically suspicious contemporaneous cases where IE was subsequently excluded) from a hospital-based group of patients referred to a cardiac department with possible infective endocarditis. Patients were identified from the echocardiography database. Cases were defined by final diagnosis. Symptoms, signs, risk factors for IE and blood investigations were recorded from case notes and examined by univariate and multivariate analyses.
Results: The sensitivity, specificity, and positive and negative predictive values of transthoracic echocardiography (TTE) for detection of IE in clinically suspected cases were 71%, 98%, 57% and 99%, respectively. Univariate analyses revealed a significant association between IE and several clinical features. Under multivariate analysis, previous heart valve surgery (OR 13.3, 90%CI 3.2–55.6), positive blood cultures (OR 17.2, 90%CI 4.9–58.8), signs of embolism (OR 11.4, 90%CI 3.0–43.5), a new, altered or changing murmur (OR 10.3, 90%CI 2.8–38.5) and splenomegaly (OR 18.2, 90%CI 3.6–90.9) were independent predictors for IE.
Discussion: Clinical features at presentation continue to be important for the diagnosis of IE. Features such as positive blood cultures, signs of embolism and a changing heart murmur should be used to guide investigation and treatment of IE prior to echocardiography, or when TTE is negative.
Introduction
Infective endocarditis (IE), as defined by the European Society of Cardiology,1 is an ‘endovascular microbial infection of cardiovascular structures’. Its incidence is estimated at between 1.9 and 6.2 infections per 100 000 general population.2–4 Despite advances in treatment, establishment of preventative strategies and earlier detection of complications, in-hospital mortality remains high, at about 20%.5,6 Perhaps the most important developments over the last 20 years are those facilitating a rapid and accurate diagnosis. Early aggressive treatment is associated with improved survival.6,7
IE is often a challenging condition to diagnose, because of the presence of non-specific clinical features at the time of presentation, and the variable medical and surgical settings in which it can occur.1 The Duke criteria8 are now widely accepted as being key to the diagnosis, but their ability to perform accurately and consistently in a range of clinical settings around the world is not well-tested. Patients who have received antibiotics prior to hospital admission may present with ‘culture-negative’ IE,9so the perceived importance of echocardiography to detect vegetations increases.
The service burden of echocardiography in many hospitals is increasing, and it may not be routinely available to a general medical team on a 24-h basis. While there is no doubt that echocardiographic imaging is a necessary management tool, the reliance on early echocardiography to diagnose IE is arguably over-emphasized. A thorough assessment of clinical features remains important in the assessment of patients with IE.
The aims of this study were to explore the use of echocardiography in the diagnosis of IE, and to examine the strength of association between clinical features and a confirmed IE diagnosis.
Methods
Patients
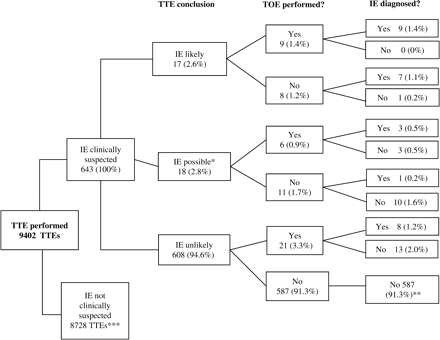
Both transthoracic (TTE) and transoesophageal (TOE) echocardiogram databases (Filemaker Pro) at our institute were used to identify IE cases and controls. These were searched systematically for studies performed during a 2-year period between July 2002 and July 2004. Each echocardiogram was categorized according to the indication for the test and the findings on the echo-report (Figure 1).
Flow diagram displaying the role of echocardiography in the diagnosis of infective endocarditis (IE). Endocarditis was defined as ‘highly likely’ if the transthoracic echocardiogram (TTE) report stated ‘endocarditis’ of a specific valve or ‘large mobile mass’; ‘possible’ if the report used the terms ‘possible endocarditis’ or ‘consider endocarditis’ and ‘unlikely’ if the report stated ‘no evidence of endocarditis’ *One set of case-notes was unobtainable. **Control patients selected from this group. ***Fourteen patients who had no clinical suspicion of IE had a suspicious lesion on TTE: 12 had the diagnosis excluded clinically; the other two had a transoesophageal echocardiogram (TOE), of whom one had confirmed IE.
Data collection
The baseline cohort comprised patients referred for a TTE with a primary indication to exclude or confirm the diagnosis of IE. From this group, all reports where IE was said to be ‘highly likely’ or ‘possible’ were identified as potential cases, and their case-records examined. Controls were taken from the 587 patients in whom the TTE report for IE was ‘unlikely’ and the final diagnosis was not IE (denoted ** in Figure 1). Each 10th patient was chosen, giving a total of 59 patients. Added to this number were the 27 patients in the other TTE categories who had IE excluded as a final diagnosis. This gave a total of 86 patients. Case notes were not available for seven of these patients, and therefore the final control group consisted of 79 patients, which were in turn combined with the 29 IE cases to form our study group of 108 patients.
Case record examination
Case records were examined for a number of pre-determined features, including demographic data, symptoms, clinical signs, risk factors for IE, examination findings and laboratory test results. Symptoms, signs and risk factors for IE were recorded as negative if they were recorded in the case notes as such, or had not been documented in the case notes. Previous dental treatment and investigations were recorded as ‘not performed’ if not documented.
Data handling and statistical analysis
Data from the case records were transferred to a spreadsheet and analysed using SPSS version 13.0. Factors from the five groups of features (symptoms, signs, risk factors, laboratory tests and other tests) were assessed using univariate and multivariate analyses.
The sensitivity, specificity and negative and positive predictive values for TTE as a predictive tool for diagnosing TTE were calculated, and corresponding 95%CIs were derived from these using Wilson's score method.10
Following tests of normality, the independent samples t-test and the Mann-Whitney U-test were used to compare means and medians, respectively, for continuous data. Where checks of distributions for continuous data revealed dissimilar shapes on comparing the IE case and control groups, data were categorized. Hence, in particular, CRP levels were categorized into two levels: 0–50 mg/l (normal or mildly raised) and ≥51 mg/l (moderately or markedly raised), and age was categorized into three levels: 20–39, 40–59 and ≥60 years.
These data were then cross-tabulated with the IE outcome data and analysed using the χ2 test of association, except in cases where <80% of the expected frequencies were of value 5 or more, when Fisher's exact test was used instead. Binary data from the original spreadsheet were analysed in an entirely similar fashion to that of the derived categorical data. Subsequent to these univariate analyses, the multivariate technique adopted was binary logistic regression.
A significance level of 5% was assumed for all univariate analyses, and a significance level of 10% was assumed at the final stage of the multivariate analysis in the identification of independent predictors of IE. The weaker significance level for the multivariate model was assumed because in a multivariate model, more stringent significant levels carry the risk of ruling out factors of clinical importance.11
The different factors were screened individually for the binary logistic regression analysis, using a significance level of 0.25 as a viable cut-off point for identifying potentially significant factors within the multivariate model (Hosmer and Lemeshow, personal correspondence).11 Using Wald's test to assess the significance of factor coefficients, the final model was then obtained using a significance level of 0.1 for inclusion of factors.
Post hoc analyses performed on the binary logistic regression model confirmed a negligible influence from outliers, and a positive 80–20 split-sample validation test result. The latter result confirmed that the model was not too conservative for use with similar cohorts, and discredited the idea that there were natural subgroups within the cohort for which an alternative model would have been more appropriate. Further, the standard errors of all model coefficients were found to be <2, thus confirming the absence of multicollinearity. The Hosmer and Lemeshow test confirmed that the model adequately fitted the data. The sensitivity and specificity of the binary logistic regression model relative to the IE outcomes within the dataset were also determined. The binomial test and Fisher's exact test were used to investigate outcomes for IE patients, while to assist in the interpretation of the univariate analyses, the Mann-Whitney U-test was used to compare ESRs across different CRP levels.
Results
Echocardiography and prediction of IE
A total of 9402 TTEs were performed in the 2-year period, of which 643 were for patients with a clinical suspicion of IE (Figure 1). Twenty-nine confirmed cases of IE were identified. Of these 29, 12 had both TTE and TOE results to support the diagnosis, eight had only TTE done (results supporting the diagnosis), and a further eight had both TTE and TOE, but only the TOE results supported the diagnosis. The remaining confirmed case of IE related to a patient with no clinical suspicion of IE, but for whom the TTE result supported the diagnosis. In patients suspected of having IE, TTE did not support the diagnosis for 608 (94.6%). The eight patients from this group who were later diagnosed with the condition had been recommended for TOE on account of continued clinical suspicion.
Therefore, of 49 patients with a TTE report suggestive of possible IE, only 21 (42.9%) were subsequently confirmed as having IE. These 21 patients comprised 20/35 patients with a previous clinical suspicion of IE and 1/14 with no such suspicion. Thus for patients clinically suspected of IE, TTE had a sensitivity of 71% (95%CI 53–85%), a specificity of 98% (95%CI 96–99%), a positive predictive value of 57% (95%CI 41–72%) and a negative predictive value of 99% (95%CI 97–99%) (Table 1).
Final diagnoses vs. transthoracic echocardiography results for the detection of bacterial endocarditis in clinically suspected cases
| . | Endocarditis diagnosed . | Endocarditis not diagnosed . | Total . |
|---|---|---|---|
| Echo ‘possible’ | 20 | 15 | 35 |
| Echo ‘unlikely’ | 8 | 600 | 608 |
| Total | 28 | 615 | 643 |
| . | Endocarditis diagnosed . | Endocarditis not diagnosed . | Total . |
|---|---|---|---|
| Echo ‘possible’ | 20 | 15 | 35 |
| Echo ‘unlikely’ | 8 | 600 | 608 |
| Total | 28 | 615 | 643 |
Final diagnoses vs. transthoracic echocardiography results for the detection of bacterial endocarditis in clinically suspected cases
| . | Endocarditis diagnosed . | Endocarditis not diagnosed . | Total . |
|---|---|---|---|
| Echo ‘possible’ | 20 | 15 | 35 |
| Echo ‘unlikely’ | 8 | 600 | 608 |
| Total | 28 | 615 | 643 |
| . | Endocarditis diagnosed . | Endocarditis not diagnosed . | Total . |
|---|---|---|---|
| Echo ‘possible’ | 20 | 15 | 35 |
| Echo ‘unlikely’ | 8 | 600 | 608 |
| Total | 28 | 615 | 643 |
Predicting a diagnosis of infective endocarditis: differences between cases and controls
Blood investigations (Table 2)
In Table 2the results of blood investigations are compared at the univariate level for IE cases and controls. The increase in ESR and white blood cell counts in cases compared with controls was highly significant. By contrast, there was a significant decrease in albumin levels for cases vs. controls. On progressing from controls to cases, neither increases in serum creatinine levels and neutrophil counts, nor the decreases in glucose concentrations, haemoglobin concentrations and platelet counts, were significant. None of the factors representative of the above measures proved to be independent predictors of confirmed IE.
Univariate analyses for factors representative of blood investigations
| Factor . | Units . | Test . | Cases . | Controls . | p . |
|---|---|---|---|---|---|
| Albumin | g/l | Mann-Whitney U-test | 31 (15–44) | 36 (15–52) | 0.026 |
| Serum creatinine | µmol/l | Mann-Whitney U-test | 91 (52–1019) | 83 (14–535) | 0.217 |
| ESR | mm/hour | Mann-Whitney U-test | 66 (9–189) | 34 (2–381) | 0.005 |
| Glucose | mmol/l | Mann-Whitney U-test | 5.6 (3.9–9.7) | 5.8 (2.8–21.3) | 0.734 |
| Neutrophil count | 109/l | Mann-Whitney U-test | 8.9 (2.9–21.0) | 7.7 (1.5–36.3) | 0.120 |
| White blood cell count | 109/l | Mann-Whitney U-test | 12.1 (5.9–30.5) | 9.5 (3.1–39.9) | 0.007 |
| Haemoglobin | g/l | Independent samples t-test (equal variances not assumed) | 115 ± 24 | 120 ± 26 | 0.400 |
| Platelet count | (109/l) | Independent samples t-test (equal variances assumed) | 236 ± 150 | 284 ± 133 | 0.113 |
| Factor . | Units . | Test . | Cases . | Controls . | p . |
|---|---|---|---|---|---|
| Albumin | g/l | Mann-Whitney U-test | 31 (15–44) | 36 (15–52) | 0.026 |
| Serum creatinine | µmol/l | Mann-Whitney U-test | 91 (52–1019) | 83 (14–535) | 0.217 |
| ESR | mm/hour | Mann-Whitney U-test | 66 (9–189) | 34 (2–381) | 0.005 |
| Glucose | mmol/l | Mann-Whitney U-test | 5.6 (3.9–9.7) | 5.8 (2.8–21.3) | 0.734 |
| Neutrophil count | 109/l | Mann-Whitney U-test | 8.9 (2.9–21.0) | 7.7 (1.5–36.3) | 0.120 |
| White blood cell count | 109/l | Mann-Whitney U-test | 12.1 (5.9–30.5) | 9.5 (3.1–39.9) | 0.007 |
| Haemoglobin | g/l | Independent samples t-test (equal variances not assumed) | 115 ± 24 | 120 ± 26 | 0.400 |
| Platelet count | (109/l) | Independent samples t-test (equal variances assumed) | 236 ± 150 | 284 ± 133 | 0.113 |
Data are medians (range) or means ± SD, as appropriate.
Univariate analyses for factors representative of blood investigations
| Factor . | Units . | Test . | Cases . | Controls . | p . |
|---|---|---|---|---|---|
| Albumin | g/l | Mann-Whitney U-test | 31 (15–44) | 36 (15–52) | 0.026 |
| Serum creatinine | µmol/l | Mann-Whitney U-test | 91 (52–1019) | 83 (14–535) | 0.217 |
| ESR | mm/hour | Mann-Whitney U-test | 66 (9–189) | 34 (2–381) | 0.005 |
| Glucose | mmol/l | Mann-Whitney U-test | 5.6 (3.9–9.7) | 5.8 (2.8–21.3) | 0.734 |
| Neutrophil count | 109/l | Mann-Whitney U-test | 8.9 (2.9–21.0) | 7.7 (1.5–36.3) | 0.120 |
| White blood cell count | 109/l | Mann-Whitney U-test | 12.1 (5.9–30.5) | 9.5 (3.1–39.9) | 0.007 |
| Haemoglobin | g/l | Independent samples t-test (equal variances not assumed) | 115 ± 24 | 120 ± 26 | 0.400 |
| Platelet count | (109/l) | Independent samples t-test (equal variances assumed) | 236 ± 150 | 284 ± 133 | 0.113 |
| Factor . | Units . | Test . | Cases . | Controls . | p . |
|---|---|---|---|---|---|
| Albumin | g/l | Mann-Whitney U-test | 31 (15–44) | 36 (15–52) | 0.026 |
| Serum creatinine | µmol/l | Mann-Whitney U-test | 91 (52–1019) | 83 (14–535) | 0.217 |
| ESR | mm/hour | Mann-Whitney U-test | 66 (9–189) | 34 (2–381) | 0.005 |
| Glucose | mmol/l | Mann-Whitney U-test | 5.6 (3.9–9.7) | 5.8 (2.8–21.3) | 0.734 |
| Neutrophil count | 109/l | Mann-Whitney U-test | 8.9 (2.9–21.0) | 7.7 (1.5–36.3) | 0.120 |
| White blood cell count | 109/l | Mann-Whitney U-test | 12.1 (5.9–30.5) | 9.5 (3.1–39.9) | 0.007 |
| Haemoglobin | g/l | Independent samples t-test (equal variances not assumed) | 115 ± 24 | 120 ± 26 | 0.400 |
| Platelet count | (109/l) | Independent samples t-test (equal variances assumed) | 236 ± 150 | 284 ± 133 | 0.113 |
Data are medians (range) or means ± SD, as appropriate.
Clinical characteristics and risk factors (Table 3)
While age was assumed to be a risk factor, there was no significant difference between cases and controls in terms of age at the univariate level (χ2 = 1.341, p = 0.511). Further, the univariate odds ratios obtained on comparing the oldest age group with that of each of the other two were both inclusive of the value 1 (Table 3), indicating a non-significant difference in the odds of confirmed IE for older subjects. At the screening stage, age was also eliminated from the multivariate model.
Univariate and multivariate analyses for factors representative of clinical characteristics and recognized risk factors
| Factor . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|
| . | p . | OR (95%CI) . | p . | Adjusted OR (90%CI)‡ . | ||
| Age (years) | 0.511 | |||||
| 60+ vs. 20–39 | 2.1 (0.7–6.7) | |||||
| 60+ vs. 40–59 | 1.5 (0.4–5.6) | |||||
| Concurrent infection | 0.041 | 2.4 (1.0–5.9) | ||||
| Congenital heart disease | 0.298* | 2.1 (0.6–7.4) | ||||
| CRP (normal or mildly raised vs. moderately or markedly raised)† | <0.0005 | 6.4 (2.2–18.5) | ||||
| Diabetes | 0.226* | 0.3 (0.1–6.0) | ||||
| ECG | 0.449 | 1.5 (0.5–3.8) | ||||
| Gender (M vs. F) | 0.920 | 1.1 (0.5–2.4) | ||||
| History of a murmur | 0.841 | 1.1 (0.5–2.6) | ||||
| History of endocarditis | 0.383* | 2.2 (0.4–10.3) | ||||
| Invasive procedure | 0.153 | 1.9 (0.8–4.8) | ||||
| IV drug abuse | 1.000* | 1.4 (0.1–15.9) | ||||
| Previous valve surgery | 0.063 | 2.4 (1.0–6.4) | 0.003 | 13.3 (3.2–55.6) | ||
| Poor dentition | 0.159* | 4.8 (0.8–30.3) | ||||
| Previous rheumatic fever | 1.000* | 1.0 (0.3–3.4) | ||||
| Factor . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|
| . | p . | OR (95%CI) . | p . | Adjusted OR (90%CI)‡ . | ||
| Age (years) | 0.511 | |||||
| 60+ vs. 20–39 | 2.1 (0.7–6.7) | |||||
| 60+ vs. 40–59 | 1.5 (0.4–5.6) | |||||
| Concurrent infection | 0.041 | 2.4 (1.0–5.9) | ||||
| Congenital heart disease | 0.298* | 2.1 (0.6–7.4) | ||||
| CRP (normal or mildly raised vs. moderately or markedly raised)† | <0.0005 | 6.4 (2.2–18.5) | ||||
| Diabetes | 0.226* | 0.3 (0.1–6.0) | ||||
| ECG | 0.449 | 1.5 (0.5–3.8) | ||||
| Gender (M vs. F) | 0.920 | 1.1 (0.5–2.4) | ||||
| History of a murmur | 0.841 | 1.1 (0.5–2.6) | ||||
| History of endocarditis | 0.383* | 2.2 (0.4–10.3) | ||||
| Invasive procedure | 0.153 | 1.9 (0.8–4.8) | ||||
| IV drug abuse | 1.000* | 1.4 (0.1–15.9) | ||||
| Previous valve surgery | 0.063 | 2.4 (1.0–6.4) | 0.003 | 13.3 (3.2–55.6) | ||
| Poor dentition | 0.159* | 4.8 (0.8–30.3) | ||||
| Previous rheumatic fever | 1.000* | 1.0 (0.3–3.4) | ||||
*Fisher's exact test used instead of χ2 test of association, as <80% of expected frequencies were >5. †Normal or mildly raised, 0–50 mg/l; moderately or markedly raised, ≥51 mg/l. ‡Results appear only for those factors which are included in the final multivariate regression model.
Univariate and multivariate analyses for factors representative of clinical characteristics and recognized risk factors
| Factor . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|
| . | p . | OR (95%CI) . | p . | Adjusted OR (90%CI)‡ . | ||
| Age (years) | 0.511 | |||||
| 60+ vs. 20–39 | 2.1 (0.7–6.7) | |||||
| 60+ vs. 40–59 | 1.5 (0.4–5.6) | |||||
| Concurrent infection | 0.041 | 2.4 (1.0–5.9) | ||||
| Congenital heart disease | 0.298* | 2.1 (0.6–7.4) | ||||
| CRP (normal or mildly raised vs. moderately or markedly raised)† | <0.0005 | 6.4 (2.2–18.5) | ||||
| Diabetes | 0.226* | 0.3 (0.1–6.0) | ||||
| ECG | 0.449 | 1.5 (0.5–3.8) | ||||
| Gender (M vs. F) | 0.920 | 1.1 (0.5–2.4) | ||||
| History of a murmur | 0.841 | 1.1 (0.5–2.6) | ||||
| History of endocarditis | 0.383* | 2.2 (0.4–10.3) | ||||
| Invasive procedure | 0.153 | 1.9 (0.8–4.8) | ||||
| IV drug abuse | 1.000* | 1.4 (0.1–15.9) | ||||
| Previous valve surgery | 0.063 | 2.4 (1.0–6.4) | 0.003 | 13.3 (3.2–55.6) | ||
| Poor dentition | 0.159* | 4.8 (0.8–30.3) | ||||
| Previous rheumatic fever | 1.000* | 1.0 (0.3–3.4) | ||||
| Factor . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|
| . | p . | OR (95%CI) . | p . | Adjusted OR (90%CI)‡ . | ||
| Age (years) | 0.511 | |||||
| 60+ vs. 20–39 | 2.1 (0.7–6.7) | |||||
| 60+ vs. 40–59 | 1.5 (0.4–5.6) | |||||
| Concurrent infection | 0.041 | 2.4 (1.0–5.9) | ||||
| Congenital heart disease | 0.298* | 2.1 (0.6–7.4) | ||||
| CRP (normal or mildly raised vs. moderately or markedly raised)† | <0.0005 | 6.4 (2.2–18.5) | ||||
| Diabetes | 0.226* | 0.3 (0.1–6.0) | ||||
| ECG | 0.449 | 1.5 (0.5–3.8) | ||||
| Gender (M vs. F) | 0.920 | 1.1 (0.5–2.4) | ||||
| History of a murmur | 0.841 | 1.1 (0.5–2.6) | ||||
| History of endocarditis | 0.383* | 2.2 (0.4–10.3) | ||||
| Invasive procedure | 0.153 | 1.9 (0.8–4.8) | ||||
| IV drug abuse | 1.000* | 1.4 (0.1–15.9) | ||||
| Previous valve surgery | 0.063 | 2.4 (1.0–6.4) | 0.003 | 13.3 (3.2–55.6) | ||
| Poor dentition | 0.159* | 4.8 (0.8–30.3) | ||||
| Previous rheumatic fever | 1.000* | 1.0 (0.3–3.4) | ||||
*Fisher's exact test used instead of χ2 test of association, as <80% of expected frequencies were >5. †Normal or mildly raised, 0–50 mg/l; moderately or markedly raised, ≥51 mg/l. ‡Results appear only for those factors which are included in the final multivariate regression model.
Significant differences between the proportions of cases and controls were established for concurrent infection, the odds of confirmed IE being approximately 2.5 times greater with concurrent infection than without (95%CI 1.027–5.882). At the multivariate level, however, concurrent infection was not an independent predictor of IE.
At the univariate level, a highly significant difference was found between the proportions of IE cases and controls with lower CRP levels. In particular, the corresponding odds of confirmed IE for patients with normal or mildly raised CRP was found to be approximately six times greater than for patients with moderately or markedly raised CRP levels (OR 6.4, 95%CI 2.2–18.5). However, CRP was not an independent predictor of IE.
No significant differences were obtained at the univariate level for any of congenital heart disease, diabetes, ECG, gender, history of a murmur, history of endocarditis, invasive procedure, IV drug abuse, previous heart valve surgery, poor dentition or previous rheumatic fever. Of these factors, one factor, previous heart valve surgery, was an independent predictor of IE. The contribution of this factor to the final regression model was highly significant (p = 0.003) and the odds of confirmed IE in patients with previous valve surgery were greatly increased in our group (adjusted OR 13.3, 95%CI 3.2–55.6).
In 63% of patients, there was no dental record in the case-notes. In the remainder, none had a prior dental procedure, but four cases and two controls had poor dentition documented.
Microbiology
Blood cultures were performed in all 29 cases, and in 24 (82.8%) an organism was isolated. The five culture-negative patients also had negative serology. Among the controls, 75 (94.9%) had blood cultures performed and the majority (68%) were negative. Staphylococci were responsible for 10 cases (34.5%) and 9 were due to Staphylococcus aureus. Streptococcal organisms, the majority of which were from the viridans group, were responsible for 10 cases (34.5%).
Symptoms and recognized clinical signs (Table 4)
Highly significant differences in the proportions of IE cases and controls were established for each of blood culture, signs of embolism, patient-reported fever, neurological symptoms, and a new altered or changing murmur, the odds of confirmed IE in each case being significantly increased in the presence of the associated symptom or sign. Of these factors, positive blood culture and new, altered or changing murmur were found to be the most important, the defining features proving to increase the odds of confirmed IE by factors of about 12.5 and 6.0 times, respectively. Further, significant differences in the proportions of IE cases and controls were established for each of fatigue, Janeway lesions, Osler's nodes or Roth's spots, malaise, splinter haemorrhages or clubbing, and weight loss, the odds of confirmed IE in each case again being significantly increased in the presence of the associated symptom or sign, with the exception of malaise, where the odds ratio was not significant (OR 3.6, 95% CI 1.0–13.0).
Univariate and multivariate analyses for symptoms and clinical signs
| Factor . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|
| . | p . | OR (95%CI)** . | p . | Adjusted OR (90%CI) . | ||
| Arthralgia | 1.000* | 1.0 (0.3–3.4) | ||||
| Positive blood culture | <0.0005 | 12.5 (3.9–40.0) | <0.0005 | 17.2 (4.9–58.8) | ||
| Signs of embolism‡ | 0.002 | 4.8 (1.7–13.2) | 0.003 | 11.4 (3.0–43.5) | ||
| Fatigue | 0.023 | 3.6 (1.3–10.5) | ||||
| Patient-reported fever‡ | 0.006* | 5.3 (1.5–19.0) | ||||
| Janeway lesions, Osler's nodes or Roth's spots | 0.018* | – | ||||
| Malaise | 0.043 | 3.6 (1.0–13.0) | ||||
| Myalgia | 0.387* | 1.6 (0.5–4.9) | ||||
| Neurological signs | 0.883 | 1.1 (0.4–2.9) | ||||
| Neurological symptoms | 0.005 | 3.8 (1.4–9.8) | ||||
| New, altered or changing murmur† | <0.0005 | 6.0 (2.1–17.5) | 0.003 | 10.3 (2.8–38.5) | ||
| Purpura | 0.063 | 2.4 (0.9–6.4) | ||||
| Splenomegaly | 0.070 | 3.0 (1.0–8.6) | 0.003 | 18.2 (3.6–90.9) | ||
| Splinter haemorrhages or clubbing | 0.021* | 3.5 (1.2–10.0) | ||||
| Weight loss | 0.017 | 2.9 (1.2–7.2) | ||||
| Factor . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|
| . | p . | OR (95%CI)** . | p . | Adjusted OR (90%CI) . | ||
| Arthralgia | 1.000* | 1.0 (0.3–3.4) | ||||
| Positive blood culture | <0.0005 | 12.5 (3.9–40.0) | <0.0005 | 17.2 (4.9–58.8) | ||
| Signs of embolism‡ | 0.002 | 4.8 (1.7–13.2) | 0.003 | 11.4 (3.0–43.5) | ||
| Fatigue | 0.023 | 3.6 (1.3–10.5) | ||||
| Patient-reported fever‡ | 0.006* | 5.3 (1.5–19.0) | ||||
| Janeway lesions, Osler's nodes or Roth's spots | 0.018* | – | ||||
| Malaise | 0.043 | 3.6 (1.0–13.0) | ||||
| Myalgia | 0.387* | 1.6 (0.5–4.9) | ||||
| Neurological signs | 0.883 | 1.1 (0.4–2.9) | ||||
| Neurological symptoms | 0.005 | 3.8 (1.4–9.8) | ||||
| New, altered or changing murmur† | <0.0005 | 6.0 (2.1–17.5) | 0.003 | 10.3 (2.8–38.5) | ||
| Purpura | 0.063 | 2.4 (0.9–6.4) | ||||
| Splenomegaly | 0.070 | 3.0 (1.0–8.6) | 0.003 | 18.2 (3.6–90.9) | ||
| Splinter haemorrhages or clubbing | 0.021* | 3.5 (1.2–10.0) | ||||
| Weight loss | 0.017 | 2.9 (1.2–7.2) | ||||
*Fisher's exact test used instead of χ2 test of association, as <80% of expected frequencies were >5.**Missing value indicates because of a zero frequency, a corresponding OR could not be calculated. †Major Duke's criterion. ‡Minor Duke's criterion.
Univariate and multivariate analyses for symptoms and clinical signs
| Factor . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|
| . | p . | OR (95%CI)** . | p . | Adjusted OR (90%CI) . | ||
| Arthralgia | 1.000* | 1.0 (0.3–3.4) | ||||
| Positive blood culture | <0.0005 | 12.5 (3.9–40.0) | <0.0005 | 17.2 (4.9–58.8) | ||
| Signs of embolism‡ | 0.002 | 4.8 (1.7–13.2) | 0.003 | 11.4 (3.0–43.5) | ||
| Fatigue | 0.023 | 3.6 (1.3–10.5) | ||||
| Patient-reported fever‡ | 0.006* | 5.3 (1.5–19.0) | ||||
| Janeway lesions, Osler's nodes or Roth's spots | 0.018* | – | ||||
| Malaise | 0.043 | 3.6 (1.0–13.0) | ||||
| Myalgia | 0.387* | 1.6 (0.5–4.9) | ||||
| Neurological signs | 0.883 | 1.1 (0.4–2.9) | ||||
| Neurological symptoms | 0.005 | 3.8 (1.4–9.8) | ||||
| New, altered or changing murmur† | <0.0005 | 6.0 (2.1–17.5) | 0.003 | 10.3 (2.8–38.5) | ||
| Purpura | 0.063 | 2.4 (0.9–6.4) | ||||
| Splenomegaly | 0.070 | 3.0 (1.0–8.6) | 0.003 | 18.2 (3.6–90.9) | ||
| Splinter haemorrhages or clubbing | 0.021* | 3.5 (1.2–10.0) | ||||
| Weight loss | 0.017 | 2.9 (1.2–7.2) | ||||
| Factor . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|
| . | p . | OR (95%CI)** . | p . | Adjusted OR (90%CI) . | ||
| Arthralgia | 1.000* | 1.0 (0.3–3.4) | ||||
| Positive blood culture | <0.0005 | 12.5 (3.9–40.0) | <0.0005 | 17.2 (4.9–58.8) | ||
| Signs of embolism‡ | 0.002 | 4.8 (1.7–13.2) | 0.003 | 11.4 (3.0–43.5) | ||
| Fatigue | 0.023 | 3.6 (1.3–10.5) | ||||
| Patient-reported fever‡ | 0.006* | 5.3 (1.5–19.0) | ||||
| Janeway lesions, Osler's nodes or Roth's spots | 0.018* | – | ||||
| Malaise | 0.043 | 3.6 (1.0–13.0) | ||||
| Myalgia | 0.387* | 1.6 (0.5–4.9) | ||||
| Neurological signs | 0.883 | 1.1 (0.4–2.9) | ||||
| Neurological symptoms | 0.005 | 3.8 (1.4–9.8) | ||||
| New, altered or changing murmur† | <0.0005 | 6.0 (2.1–17.5) | 0.003 | 10.3 (2.8–38.5) | ||
| Purpura | 0.063 | 2.4 (0.9–6.4) | ||||
| Splenomegaly | 0.070 | 3.0 (1.0–8.6) | 0.003 | 18.2 (3.6–90.9) | ||
| Splinter haemorrhages or clubbing | 0.021* | 3.5 (1.2–10.0) | ||||
| Weight loss | 0.017 | 2.9 (1.2–7.2) | ||||
*Fisher's exact test used instead of χ2 test of association, as <80% of expected frequencies were >5.**Missing value indicates because of a zero frequency, a corresponding OR could not be calculated. †Major Duke's criterion. ‡Minor Duke's criterion.
No significant differences were obtained, however, at the univariate level for arthralgia, myalgia, neurological signs, purpura or splenomegaly.
Independent predictors of confirmed IE were confined to factors that had been recognized as predictors at the univariate level: positive blood culture, embolism, new altered or changing murmur and splenomegaly.
Outcome of patients with endocarditis
In this small series, in-hospital mortality from IE was 27.6%. Staphylococcus aureus was isolated in 62.5% of those who died. There were no deaths in the culture-negative group. Native valves were involved more than prosthetic valves (72.4 vs. 27.6%, p = 0.016), and the native mitral valve was most commonly affected (41.4%). More patients with native valve endocarditis died, compared with prosthetic endocarditis (33.0% vs. 12.5%), but this difference was not statistically significant (p = 0.38). The median length of hospital stay was 43 days (range 29–110) for those who survived, and 52 days (range 9–145) for those who died. The median length of stay for controls was significantly shorter, at 13 days (range 1–96).
Discussion
The widely acknowledged clinical features of infective endocarditis remained strongly predictive of the diagnosis in our patients. The Duke criteria were highly predictive of IE (Table 4), although it was impossible to establish a good multivariate model fit on inclusion of patient-reported fever. The latter result is probably due to the very low number (n = 3) of IE cases in the absence of patient-reported fever. With a larger cohort, it is very likely that this factor would have made for a better fit within a binary logistic regression model. Nevertheless, these findings still add considerable weight to the notion that a provisional diagnosis of IE can be made on clinical grounds without the immediate need for echocardiography.
Diagnostic value of TTE
Early use of TTE may allow rapid diagnosis of IE,12 but care must be taken to avoid over-reliance on this technique. For clinically suspected cases in our group, TTE had a sensitivity, specificity and negative predictive value of 71%, 98% and 99%, respectively, but a positive predictive value of only 57%, indicating that based on the TTE result alone, 43% of patients would have been wrongly diagnosed as genuine IE cases.
These findings suggest, in keeping with those of other studies,13,14 that TTE alone is not an ideal method by which to diagnose IE. Nevertheless, although TOE also has a specificity of >90%,15 TTE remains the most appropriate first-line imaging investigation, as it is safe, relatively simple and inexpensive.16 In the two-year period of this study, 643 patients (6.8% of all echocardiograms performed during the study period) had a TTE performed on suspicion of IE, but only 29 (4%) actually had IE. As so few patients suspected of having IE are eventually diagnosed with the condition, perhaps echocardiography resources could be used more efficiently and effectively if clinical features were carefully considered first. This might avoid the over-zealous demand for urgent echocardiography, especially given that a false negative result could delay appropriate and potentially life-saving treatment.
Blood cultures
In many developed countries, IE can present with a long period of illness prior to hospital admission. Such patients may have already been prescribed antibiotics. In our study, 4/5 culture-negative cases had documented use of antibiotics prior to hospital admission. Negative blood cultures, said to occur in 2.5–31% of all IE cases, are associated with a delay in diagnosis.17,37 As in other studies,7,9,38 streptococcus was the most common organism identified in our patients. Staphylococcus aureus was the next most prevalent, and was responsible for the greatest number of deaths similar to previous reports.39 Interestingly, a higher proportion of deaths occurred when native valves were infected in our series, despite prosthetic valve patients being generally considered to be at higher risk from Staphylococcus aureus.40 More generally, positive blood culture was highly predictive of IE, associated with an approximately 17-fold odds increase at the multivariate level (95%CI 4.9–58.8).
Clinical features
Congenital abnormalities,22–24 previous endocarditis25,26 and valvular disease25,27,28 are typically associated with increased risk of IE. However, these risk factors did not differentiate between controls and cases in our cohort. Indeed, concurrent infection was the only significant exogenous risk factor predictive of IE. It is also commonly held that recent oral or dental procedures are associated with a higher risk of IE.29 Surprisingly, none of the cases in this study reported a previous recent dental procedure and so, although the associated risk cannot be assessed, perhaps dental treatment is not as common an aetiological factor as is often stated. However, poor oral hygiene, present in four cases, may be responsible for infection30 since, in such patients, even chewing can cause bacteraemia.31
Although the presence of a new, changing or altered murmur has been reported in as few as 40% of IE patients,32 it was identified in 82.8% of IE cases in this study. This factor was a very strong independent IE predictor in our patients, with an odds ratio of ∼10.
The presence of Janeway lesions, Osler's nodes or Roth's spots, although rare, is classically regarded as an excellent clue to the diagnosis of IE.34 Recent evidence, however, has suggested that Roth's spots, previously thought to represent bacterial abscesses, should no longer be considered pathognomonic for IE.33,34 These three features were present in only 3 (10.2%) IE cases in this study, and in none of the controls. Thus, while significant at the univariate level, the very low frequencies on cross-tabulation with final diagnosis excluded these factors from the multivariate model.
Non-specific clinical signs of IE are frustrating: in general, non-specific signs are common, while highly specific signs are rare, and many of the symptoms commonly found in IE are ubiquitous in the population. For example, fatigue had an OR for IE of ∼3.5, but at any one time, 18.3% of the general population report substantial fatigue lasting >6 months.21 Nevertheless, such discrepancies may be due to confounding, since as a predictive factor fatigue was lost at the screening stage on building the multivariate model.
One might expect a fall in CRP levels to <50 mg/l to be associated with a lower incidence of IE. However, persistently or highly raised CRP may have been a clinical reason for requesting an echocardiogram to exclude IE. The predictive status of CRP for our cohort at the univariate level may therefore have been influenced by confounding. However, ESR was significantly higher in IE cases than in controls.
Importance ranking
The adjusted odds ratios forthcoming from the multivariate model are suggestive of the following importance ranking for significant factors: splenomegaly > positive blood culture > previous valve surgery > signs of embolism > new, altered or changing murmur. However, the wide confidence intervals for these factors indicate the need to treat this ranking with caution. Nevertheless, the recognition of previous valve surgery as a critical independent factor is consistent with clinical findings, lending credit to the value of our multivariate model.
General limitations
The sensitivity and specificity for the logistic regression model were 62% and 96%. More generally, the model was able to correct predict outcomes for 86.5% of the data, and the Hosmer and Lemeshow test confirmed a good model fit (χ2 = 2.661, p = 0.914). Nevertheless, some of our findings at the univariate and multivariate levels should be interpreted with caution.
In particular, this was a retrospective study and it was thus inevitable that some data would be missing from the case notes. ‘Missing data’ may have been due either to investigations not being performed or, alternatively, being performed but not recorded in the case notes. Decisions regarding data interpretation were necessary in order to compile a dataset suitable for analysis. Cases and controls were identified from our echocardiography database, and we may have missed some cases that were not recorded in this system. The incidence of IE is generally low in the population, and the relatively small numbers in our study result in wide confidence intervals for some parameters. Thus, for example, the failure to detect previous valve surgery as a significant factor at the univariate level, given its recognition as an independent predictive factor at the multivariate level, should be interpreted in the light of sample size limitations and in particular, the conservative degree of departure from significance at the univariate level (here p = 0.063).
Conclusions
In the UK National Health Service, the service burden of echocardiography is high, and includes a large number of requests for suspected IE where the yield of positive diagnoses is low. This study has confirmed that key clinical features at presentation remain strongly associated with a diagnosis of IE, even in the modern era. Our findings support a strategy of careful clinical assessment in a patient with suspected IE, which would ensure more appropriate and efficient use of echocardiography services.
The authors gratefully acknowledge the permission of Robert Newcombe (Professor of Medical Statistics, Cardiff University) to use his spreadsheet in the derivation of confidence intervals by means of Wilson's score method, together with his words of wisdom on choice of methodology for calculating confidence intervals.
References
The Task Force on Infective Endocarditis of the European Society of Cardiology. Guidelines on Prevention, Diagnosis and Treatment of Infective Endocarditis.
Van der Meer JT, Thompson J, Valkenburg HA, Michel MF. Epidemiology of bacterial endocarditis in the Netherlands. 1. Patient Characteristics.
Young AE. Aetiology and epidemiology of infectious endocarditis in England and Wales.
Berlin JA, Abrutyn E, Strom BL. Incidence of infective endocarditis in the Delaware Valley 1988–1990.
Mansur AJ, Grinberg M, Cardoso RH. Determinants of prognosis in 300 episodes of infective endocarditis.
Netzer RO, Zollinger E, Seiler C, Cerny A. Infective endocarditis: clinical spectrum, presentation and outcome. An analysis of 212 cases 1980–1995.
Netzer RO, Altwegg SC, Zollinger E, Tauber M, Carrel C, Seiler C. Infective endocarditis: determinants of long term outcome.
Durack DT, Lukes AS, Bright DK. New criteria for the diagnosis of infective endocarditis: utilisation of specific echocardiographic findings. Duke Endocarditis Service.
Wallace SM, Walton BI, Kharbanda RK, Hardy R, Wilson AP, Swanton RH. Mortality from infective endocarditis: clinical predictors of outcome.
Wilson, EB. Probable inference, the law of succession, and statistical inference.
Kuruppu JC, Corretti M, Mackowiak P, Roghmann M-C. Overuse of transthoracic echocardiography in the diagnosis of native valve endocarditis.
Jaffe WM, Morgan DE, Pearlman AS. Infective endocarditis, 1983–1988: echocardiographic findings and factors influencing morbidity and mortality.
Mügge A, Daniel WG, Frank G. Echocardiography in infective endocarditis: reassessment of prognostic implications of vegetation size determined by the transthoracic and the transesophageal approach.
Flachskampf FA, Daniel WG. Role of transoesophageal echocardiography in infective endocarditis.
Naber CK, Erbel R. Diagnosis of culture negative endocarditis: novel strategies to prove the suspect guilty.
Black N, Moore L. Comparative audit between hospitals: the example of appendectomy.
McKee M, Dixon J, Chenet L. Making routine data adequate to support clinical audit.
Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJM, Wessely SC. Population based study of fatigue and psychological distress.
Johnson DH, Rosenthal A, Nadas AS. A forty year review of bacterial endocarditis in infancy and childhood.
Corone P, Levy A, Hallali P. A propos de 54 cas d’endocarditis infectieuses observes en 32 ans sur une population de 2038 cardiopathies congenitale.
Li W, Somerville J. Infectious endocarditis in the grown-up congenital heart (GUCH) population.
Michel PR, Acar J. Native cardiac disease predisposing to infectious endocarditis.
Leport C. The Endocarditis Working Group of the International Society of Chemotherapy. Antibiotic prophylaxis for infective endocarditis.
Calderwood SB, Swinski LA, Waternaux CM. Risk factors for the development of prosthetic valve endocarditis.
Leport C, Vilde JL, Bricaire F. Fifty cases of late prosthetic valve endocarditis: improvements in prognosis over a 15 year period.
Rechmann P, Seewald M, Thomas L. Untersuchungen zur Bakteriamie bei zahnarztlichen Eingriffen.
Deacon JM, Pagliaro AJ, Zelicof SB, Horowitz HW. Current Concepts Review – Prophylactic Use of Antibiotics for Procedures after Total Joint Replacement.
Stanboulian D, Carbone E. Recognition, management and prophylaxis of endocarditis.
Bonow RO, et al. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease).
Farrior JB, Silverman ME. A consideration of the differences between a Janeway's lesion and an Osler's node in infectious endocarditis.
Prendergast BD. Diagnostic criteria and problems in infective endocarditis.
Working Party of the British Society for Antimicrobial Therapy. Guidelines: Antibiotic treatment of streptococcal, enterococcal and staphylococcal endocarditis.
Hricak V, Kovacik J, Marks P, West D, Kromery V Jr. Aetiology and outcome in 53 cases of native valve staphylococcal endocarditis.
Author notes
From the 1University of Edinburgh, Cardiology Department, Western General Hospital, and 2Public Health Sciences, University of Edinburgh Medical School, Edinburgh, UK