-
PDF
- Split View
-
Views
-
Cite
Cite
Robin J Marles, Amy L Roe, Hellen A Oketch-Rabah, US Pharmacopeial Convention safety evaluation of menaquinone-7, a form of vitamin K, Nutrition Reviews, Volume 75, Issue 7, July 2017, Pages 553–578, https://doi.org/10.1093/nutrit/nux022
Close - Share Icon Share
Abstract
Vitamin K plays important biological roles in maintaining normal blood coagulation, bone mineralization, soft tissue physiology, and neurological development. Menaquinone-7 is a form of vitamin K2 that occurs naturally in some animal-derived and fermented foods. It is also available as an ingredient of dietary supplements. Menaquinone-7 has greater bioavailability than other forms of vitamin K, which has led to increasing sales and use of menaquinone-7 supplements. This special article reviews the chemistry, nomenclature, dietary sources, intake levels, and pharmacokinetics of menaquinones, along with the nonclinical toxicity data available and the data on clinical outcomes related to safety (adverse events). In conclusion, the data reviewed indicate that menaquinone-7, when ingested as a dietary supplement, is not associated with any serious risk to health or with other public health concerns. On the basis of this conclusion, US Pharmacopeia monographs have been developed to establish quality standards for menaquinone-7 as a dietary ingredient and as a dietary supplement in various dosage forms.
INTRODUCTION
Vitamin K1 (phylloquinone) is the major form of vitamin K in the diet of humans. It is consumed as a nutrient in green leafy vegetables. Vitamin K2 (menaquinone, or MK) is obtained through the consumption of meat, dairy products, and fermented foods and from bacteria in the large intestine. Menaquinone-7 (MK-7) is a form of vitamin K2 found in certain fermented or cultured vegetables and dairy foods as well as in dietary supplements. Natto, a fermented soybean food, is a major dietary source of MK-7 and has been consumed in Japan for centuries.1 The use of MK-7 as a source of vitamin K is a recent development driven largely by the results of studies demonstrating that MK-7 has superior bioavailability over other menaquinone homologs and vitamin K1.2,3
The US Pharmacopeial (USP) Convention publishes compendia of quality standards such as the US Pharmacopeia (USP), the National Formulary (NF), and the Food Chemicals Codex that have legal standing in the United States, Canada, and many other countries. The USP–NF is a compilation of the USP and the NF. Monographs for dietary supplements are published as separate sections in the USP–NF. In order to determine the suitability of dietary ingredients and dietary supplements in dosage forms for admission to the monograph development process, the USP Dietary Supplements Admission Evaluations Joint Standard Setting Subcommittee performs an admission evaluation. Summaries of the admission criteria and of the admission evaluation supporting the development of the MK-7 monographs were published in the Dietary Supplements Compendium 2015.4 This article provides a more comprehensive and updated review of MK-7 and presents the available evidence supporting the safety of MK-7 as an ingredient of dietary supplements.
METHODS
This narrative review is based on a comprehensive search of the peer-reviewed scientific literature published up to July 2016. Human evidence reviewed includes clinical safety studies, clinical studies of MK-7 pharmacodynamics and pharmacokinetics, and studies investigating nutritional requirements for vitamin K, exposure to vitamin K through dietary and supplemental intake, postmarket surveillance, adverse events related to vitamin K intake, and interactions between vitamin K and drugs or other dietary supplements. In vivo animal studies and in vitro experiments that investigated potential risks to human health, including genotoxicity, carcinogenicity, and reproductive and developmental toxicity, as well as animal and in vitro studies of pharmacokinetics, mechanisms of action, and effects on target organs were also considered. Information from animal studies regarding the no observed adverse effect level (NOAEL) or the lowest observed adverse effect level (LOAEL) was used to determine the relative safety margin for the use of MK-7 as a dietary ingredient. These scientific data were reviewed in the context of MK-7’s regulatory status and contemporaneous extent of use globally, including product-label-recommended intake levels.
BACKGROUND
Vitamin K chemical structures and nomenclature
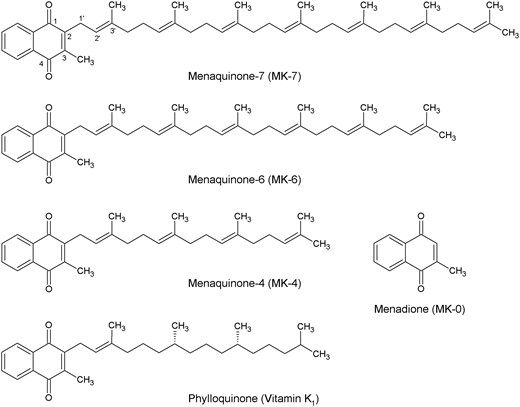
The collective term vitamin K encompasses lipid-soluble substances that possess carboxylase enzyme cofactor activity for the conversion of glutamate residues to γ-carboxyglutamate (Gla) residues. These substances have a common 3-methyl-1,4-naphthoquinone structure, called menadione, with a side chain comprised of a variable number of isoprenoid units at the 2-position (Figure 1). The K is derived from the German word Koagulation, since vitamin K became known as the coagulation vitamin after research into the cause of bleeding tendencies in chickens fed a diet that was low in both fat and what is now known as vitamin K.5 Naturally occurring forms of vitamin K include phylloquinone (listed as vitamin K1 in the US Pharmacopeia and named “phytonadione” by the US Adopted Names [USAN] Council) and the MK series (collectively referred to as vitamin K2).6
Menaquinone nomenclature is based on the number of isoprenoid units and the degree of saturation in the side chain, which varies with the organism by which the vitamin K homolog is biosynthesized and ranges from 1 to 15 (eg, MK-7 for the vitamer with a side chain consisting of 7 isoprenoid units). An alternate but superseded nomenclature system has the name “vitamin K2” followed parenthetically by the number of carbons in the side chain (eg, vitamin K2(35) was a synonym for MK-7 because it has 7 × 5 carbon isoprenoid units).
The International Union of Pure and Applied Chemistry’s systematic chemical name for the naturally occurring isomer (all-E)-MK-7, also known as (all-trans)-MK-7, is 2-[(2E,6E,10E,14E,18E,22E)-3,7,11,15,19,23,27-heptamethyloctacosa-2,6,10,14,18,22,26-heptaenyl]-3-methyl-1,4-naphthalenedione. The molecular formula is C46H64O2, the molecular weight is 649.00, and the Chemical Abstracts Service (CAS) number is 2124-57-4.7
Some bacteria produce menaquinone analogs in which 1 or more of the isoprenoid units is saturated. For example, tetrahydromenaquinone-9, abbreviated MK-9(H4), is found in cheeses such as Norwegian Jarlsberg and Swiss Emmental, which are fermented by propionibacteria.6
Phylloquinone has a phytyl side chain comprised of 4 isoprenoid units, of which the terminal 3 are saturated (Figure 1). It is noteworthy that chlorophyll has the same side chain.6 Hydrogenation of phylloquinone-rich vegetable oils produces appreciable amounts of 2′,3′-dihydrophylloquinone. In rats, this substance has some vitamin K activity, as evidenced by inhibition of both warfarin-induced prolongation of blood coagulation and warfarin-induced decrease of serum total osteocalcin levels.3
Menadione (MK-0, vitamin K3), which lacks the isoprenoid side chain, is a synthetic vitamin K homolog. Natural occurrence of this compound in a trace amount in Staphylococcus aureus has been reported.8 It has been suggested that menadione is a biosynthetic precursor of other forms of vitamin K since radiolabeled menadione is incorporated into menaquinones in some species, but this has not been demonstrated conclusively.8 The sale of synthetic menadione as a prescription drug has been discontinued in some countries because of reported adverse effects of hemolysis and liver toxicity when administered in therapeutic doses.9,10
Demethylmenaquinones, which are menaquinones without the methyl group at the 3-position on the naphthoquinone moiety, also have been identified in nature11 as biosynthetic precursors to the menaquinones.8
Other synthetic drug forms of vitamin K include the following: menadiol diacetate, dibutyrate, sodium diphosphate and sodium disulfate derivatives (vitamin K4), 4-amino-2-methyl-1-naphthol (vitamin K5), 1,4-diamino-2-methylnaphthalene (vitamin K6), and 4-amino-3-methyl-1-naphthol (vitamin K7).8
Natural sources of menaquinones
Long-chain menaquinones are biosynthesized primarily by bacteria, which include aerobic, facultative anaerobic, and obligate anaerobic species of many genera such as Actinomyces, Bacillus, Bacteroides, Corynebacterium, Escherichia, Lactobacillus, Lactococcus, Leuconostoc, Propionibacterium, Staphylococcus, Streptococcus, and Vibrio. These bacteria occur in the environment, as food fermentation species, and as part of animal intestinal microflora.8,11,12 Bacteria use menaquinones as electron carriers in their respiratory chain, analogous to the function of the ubiquinones (also known as coenzyme quinones, abbreviated as CoQ) in other bacteria, plant, fungal, and animal cells. Ubiquinones also have a polyisoprenoid side chain of variable length (eg, 1 with 10 isoprenoid units, thus called CoQ10 or ubidecarenone, is metabolically important in humans).8,13
Menaquinones with a side chain composed of 1 to 13 isoprene units are commonly encountered in animal tissues.6,14 Menaquinone-7 has been detected in some samples of human milk. For example, in 40% of analyzed samples from Italian women, MK-7 was detected at levels ranging from the limit of detection to 4.6 ng/mL; in Japanese women, it was detected at levels up to 1.675 ng/mL, depending on dietary intake levels.15 Menaquinone-14 and MK-15 have been identified in the feces of humans, cynomolgus macaque monkeys, and rabbits.16 Menaquinone-14 has also been identified in bovine and porcine liver and spleen.17 This indicates that long-chain menaquinones from the diet as well as from the intestinal microflora are taken up into host tissues.
Menaquinone-4 is generally considered to be of animal rather than bacterial origin.14 However, small amounts (5 ng/mL) have been isolated, along with MK-7 (20 ng/mL), from the supernatant of Bacillus subtilis cultures and other bacteria.18 In animals, MK-4 is biosynthesized in extrahepatic tissues (eg, brain, kidney, pancreas, fat, reproductive organs, and salivary gland) from phylloquinone and, to some extent, from long-chain menaquinones.14
Phylloquinone is the only form of vitamin K found in plants and in cyanobacteria at significant levels and is the major form of vitamin K in the human diet.6,14 Green leafy vegetables such as kale, collards, spinach, turnip greens, beet greens, Swiss chard, and rapini contain between 300 µg and 900 µg of vitamin K1 per 100 g. Soybean, canola/rapeseed, and olive oils contain 50–200 µg/100 g. Other vegetable oils such as peanut, corn, sunflower, and safflower contain 1–10 µg/100 g.19–21
Fungi do not produce menaquinones, although they do produce other 1,4-naphthoquinones.8
Commercial production of MK-7
Options for commercial production of (all-E)-MK-7 include chemical synthesis (eg, by the method of Baj et al22) and fermentation of soybean protein concentrate (soybean peptones)23–25 using strains of B subtilis subsp subtilis (Ehrenberg) Nakamura et al capable of fermenting soybeans to produce natto26 (for that reason, those bacterial strains formerly classified as Bacillus natto Sawamura were subsequently classified as B subtilis subsp natto).18
Extraction and purification of MK-7 from the fermentation broth to produce the dietary ingredient described in the USP–NF MK-7 monograph27 provides a substance consisting of not less than (NLT) 96% and not more than (NMT) 101% of MK-7 and NMT 2% of MK-6. The USP–NF also has monographs for other preparations and dosage forms of MK-7. The B. subtilis subsp subtilis Menaquinone Extract monograph sets out standards for a less refined supercritical carbon dioxide extract, a brown oil consisting mainly of fat (>97%) that contains NLT 1.5% and NMT 5% of MK-7 and NLT 0.014% and NMT 0.15% of MK-6. The MK-7 Preparation monograph sets out standards for a manufacturing intermediate consisting of concentrated MK-7 extract, along with 1 or more inert substances, in a solid or liquid form, that contains NLT 90% and NMT 120% of the labeled amount (ppm) of MK-7. There are also monographs for MK-7 capsules and MK-7 tablets.27
Isomerization of the predominant natural (all-E)-MK-7 to the mono-cis form, which has the 2Z, instead of the 2E, configuration with respect to the first double bond and the 3′ methyl group of the side chain, may occur as a result of exposure to light or as a byproduct of synthetic production. Phylloquinone’s cis form is retained longer in the liver but has little or no biological activity.28,29 This has been extrapolated to a probably reasonable assumption of the greater biological importance of (all-E)-MK-7 compared with cis-MK-7 (although a search of the literature revealed no experimental confirmation of this assumption). Consequently, specific synthetic22,30 methods have been developed for (all-E)-MK-7, and USP has established quality specifications that include analytical procedures and the acceptance criterion of NMT 2% of cis-MK-7.27
Different methods of preparation of the MK-7 used in clinical studies to date have resulted in differences in investigational products, which are not always described in detail. For example, Schurgers et al2 described the MK-7 they used only as a natural form prepared by extraction from natto food. Conly et al31 obtained a mix of menaquinones (92% MK-7 and MK-8 with small amounts of MK-4, MK-5, MK-6, and MK-9 and a trace of MK-10) from S aureus ATCC 29213 fermentation of trypticase soy broth and yeast extract.
SAFETY OF MK-7
To evaluate the safety of MK-7 as an ingredient in dietary supplements, the essential biological roles of vitamin K in its various forms will be described, along with the hazards to health of an insufficient intake, or deficiency, of vitamin K. The available evidence for consumers’ levels of exposure to vitamin K, and to MK-7 in particular, from foods and supplements will then be evaluated.
Biological roles of vitamin K
In humans, vitamin K is a cofactor for a specific carboxylase enzyme that transforms selective glutamate residues to Gla residues. Seventeen Gla proteins have been identified to date, and vitamin K–dependent carboxylation is essential to their function.32 γ-Carboxyglutamate proteins synthesized in the liver help maintain normal blood coagulation through a balance of both procoagulant factors (II, VII, IX, and X) and anticoagulant proteins (C and S). Vitamin K–dependent proteins synthesized by other tissues include osteocalcin protein, which is involved in the formation of bone, and matrix Gla protein, which is formed in blood vessel walls, other soft tissues, cartilage, and bone and has been shown to act as a potent inhibitor of soft tissue calcification.6,33–37
The roles of vitamin K in bone health are mediated to a large extent by 4 vitamin K–dependent proteins and require the presence of adequate vitamin D and calcium. The complexity of these interactions and other dietary, lifestyle, ethnicity, sex, and genetic factors among people and the differences in outcome measures between clinical studies may be sources of some of the conflicting evidence in the literature38–44 regarding the role of vitamin K in increasing bone strength and reducing the risk of fractures.
Osteocalcin, expressed in osteoblasts upon the onset of bone mineralization and eventually becoming the second most abundant protein (after collagen) in the bone matrix, binds calcium ions weakly and hydroxyapatite strongly, allowing it to bind mineralized bone matrix when it is carboxylated.45 It is also found in dentin. There is no homology with vitamin K–dependent plasma proteins involved in blood coagulation regulation.46 The transcription and translation of osteocalcin is regulated by 1,25-dihydroxyvitamin D47; carboxylation of osteocalcin to activate the binding of calcium is stimulated by vitamin K and dihydroxyvitamin D and is inhibited by warfarin treatment. In human bone, osteocalcin is not fully carboxylated at each of its Gla sites, and much of the osteocalcin in human plasma is described as undercarboxylated. To support full carboxylation, one would need an intake of vitamin K far in excess of what is thought to be nutritionally required, eg, up to 5 mg/d. This indicates that while osteocalcin undercarboxylation may reflect low vitamin K intake and be associated with dysfunctional bone remodeling and other disorders of bone resorption and mineralization,45 and while supplementation with vitamin K can increase the ratio of carboxylated osteocalcin to undercarboxylated osteocalcin significantly and in a dose-dependent manner (eg, using MK-7 at doses of 100–200 µg/d for 4–12 weeks48), full carboxylation of all of the Gla sites on osteocalcin may not be the normal state in humans. This differs from many animal models in which full carboxylation of osteocalcin is seen, resulting in challenges for the interpretation of experiments with vitamin K in animals.39,45 For example, the Institute of Medicine’s (IOM’s) Adequate Intake levels of vitamin K (discussed in greater detail below) were based on median intake measures of the nutrient rather than a particular physiological function,9 but it is now recognized that polymorphisms in the gene for vitamin K–dependent γ-glutamyl carboxylase (GGCX) and for the vitamin K epoxide reductase complex subunit 1 gene (VKORC1) cause significant changes in the correlation between the level of serum vitamin K and the ratio of undercarboxylated osteocalcin to fully carboxylated osteocalcin in humans.45,47,49
The osteoblasts expressing osteocalcin are the key bone-forming cells taking calcium from the blood circulation and binding it to the bone matrix. However, bone resorption by osteoclasts that adhere to the bone matrix and then secrete acid and lytic enzymes to degrade bone is also an essential aspect of bone growth and remodeling. Osteoclasts are specialized cells derived from the monocyte/macrophage hematopoietic lineage and whose differentiation, activation, and survival are controlled by a family of biologically related proteins, including macrophage colony-stimulating factor, interleukin 1, tumor necrosis factor α, osteoprotegerin, receptor activator of nuclear factor–kappa B (RANK), and RANK ligand (RANKL).50–52
The appropriate balance between osteoblast activity and osteoclast activity is necessary for good bone health, with many skeletal diseases in adults (eg, osteoporosis, periodontal disease, rheumatoid arthritis, multiple myeloma, and some metastatic cancers) attributable, at least in part, to excess osteoclastic activity leading to an imbalance in bone remodeling that favors resorption.51 Bone remodeling normally results in replacement of the entire skeleton every 7 to 10 years,5 and the rate triples from age 50 to 65 in typical women.53 Remodeling can improve bone strength by repairing acquired defects, but homeostatic remodeling, while necessary to maintain blood calcium levels when calcium and vitamin D intake are not sufficient, contributes structural weakness to bone through osteoporosis.53 Osteocalcin may function in the regulation of bone mineral maturation as an attractant for osteoclast progenitor cells.45 Monocytes and macrophages are capable of resorbing bone; osteocalcin-deficient bone is poorly resorbed in vivo; and in vitro studies showed that osteocalcin-deficient bone is ineffective in attracting monocytes, while purified osteocalcin is an excellent chemoattractant for human peripheral blood monocytes. All of these findings support the hypothesis that osteocalcin plays a role in osteoclast-mediated bone resorption.46
Matrix Gla protein is another vitamin K–dependent protein structurally related to osteocalcin, with 5 Gla residues that can bind calcium’ ions. It is expressed by chondrocytes, vascular smooth muscle cells, endothelial cells, and fibroblasts. Two post-translational modifications to matrix Gla protein, serine phosphorylation and glutamate carboxylation, result in the various forms found both in the circulation and in tissues: nonphosphorylated (desphospho-), phosphorylated, uncarboxylated, and carboxylated matrix Gla protein.54 Owing to the more widespread tissue distribution of vitamin K2 compared with vitamin K1, the menaquinones may be more specifically involved in the γ-carboxylation required for activation of matrix Gla protein,55 which binds calcium crystals, inhibits crystal growth, inhibits hydroxyapatite formation, binds and inactivates bone morphogenetic protein 2, and may have other roles related to vascular calcification.54 Activated matrix Gla protein prevents vascular smooth muscle cell osteogenic conversion and mineralization, and, by inhibiting the deposition of calcium in the vessel walls, exerts a protective effect against arterial calcification, atherosclerosis, and arterial stiffening.54 Therefore, the balance between carboxylated and uncarboxylated matrix Gla protein is very important, with the plasma undercarboxylated fraction of matrix Gla protein considered a marker of vascular vitamin K status and high levels of desphospho-undercarboxylated matrix Gla protein considered a risk marker for cardiovascular morbidity and mortality.55,56 Again, calcium, vitamin D, and vitamin K2 are required, in the correct proportions, for good health.5
Protein S, synthesized by osteoblasts, is a vitamin K–dependent protein with a thrombin-sensitive region, an epidermal growth factor–like domain, and a steroid hormone–binding domain. A role in bone function was suggested after findings of severe osteopenia, low bone mineral density, and vertebral compression fractures in pediatric cases with very low protein S levels.45 Protein S has significant homology with a vitamin K–dependent growth arrest–specific gene product, gas6, found in chondrocytes and shown to regulate osteoclast activity.39
Periostin is a vitamin K–dependent matricellular protein produced and secreted by bone-derived mesenchymal stromal cells. It is expressed in collagen-rich connective tissues, including bone, and is abundant in mineralized bone nodules. It is believed to function in the formation of the extracellular bone matrix.45,47
With regard to genetic interactions, vitamin K2 also has a transcriptional regulatory function, binding to and activating the orphan nuclear steroid and xenobiotic receptor (SXR) and inducing expression of its target gene, CYP3A4. In an osteosarcoma cell line, vitamin K2 increased mRNA levels of the osteoblast markers bone alkaline phosphatase, osteoprotegerin, osteopontin, and matrix Gla protein.57 Individuals who carry the APOE-ɛ4 allele have rapid hepatic clearance of chylomicron remnants and lower serum cholesterol and triglycerides. They have also been reported to have lower bone mineral density and increased risk of fracture, which has been attributed to inadequate transport of vitamin K to the skeletal tissue, but evidence for this association between genotype and bone loss effect is inconsistent.40
Additional potential mechanisms by which vitamin K2 may affect bone metabolism, some of which may not involve glutamyl residue carboxylation, include reducing urinary calcium excretion and inhibiting bone-resorbing agents such as prostaglandin E2 and interleukin58; increasing insulinlike growth factor 1, growth differentiation factor 15, and stanniocalcin 2 levels; reducing the proapoptotic proteins Fas and Bax in osteoblasts; and decreasing osteoclast differentiation by increasing osteoprotegerin and reducing RANKL. Many of these potential mechanisms have been studied in vitro or in vivo and require further study in humans, taking into account calcium and vitamin D intake as well as other factors.59
The main dietary source of vitamin K is phylloquinone from green leafy and cruciferous vegetables, which are also sources of potassium and magnesium, healthy intakes of which have been associated with increased bone mineral density.39 While natto is an important dietary source of MK-7, it must be noted that other components of natto may also contribute to improved bone mineral density.14 Kaneki et al60 present evidence suggesting that the contribution of isoflavonoids, one of the other soy ingredients with a potential role in bone health, might not be significant at the levels of soy products consumed, although Ikeda et al61 argue in favor of a potential contributory role for soy isoflavones, which are abundant and bioavailable in natto.
Vitamin K supplementation studies should be considered in the context of beneficial effects of vitamin K above those attained by calcium and vitamin D supplementation alone,39 particularly since studies of supplements containing only vitamin K found little evidence of significant improvements in bone mineral density.62 Chan et al63 found no association between dietary vitamin K intake and fracture risk in Chinese community-dwelling older men and women but noted that their population sample had a high average dietary vitamin K intake that may have limited their ability to detect such an association. Osteocalcin production is regulated by retinoic acid, estrogens, glucocorticoids, and 1,25-dihydroxyvitamin D, and there is increasing evidence for synergy between vitamins D and K with regard to reducing the risk of bone fracture and improving bone quality.64 Forli et al65 reported potentially favorable effects of MK-7 supplementation on lumbar spine bone mineral density in patients after lung or heart transplant but noted the confounding effect of an increase in parathyroid hormone levels in the treatment group, indicating an insufficient vitamin D status in many of the patients. Kanellakis et al66 noted that supplementation with a combination of calcium, vitamin D, and vitamin K2 (MK-7) also resulted in an increase in the serum level of insulinlike growth factor 1, an anabolic hormone-like peptide reported to stimulate bone formation in postmenopausal women, apparently via osteoblast differentiation, although they found no effect of the supplementation on osteoprotegerin or the osteoclastogenesis inducer RANKL.
Knapen et al67 noted that vitamin K2 (MK-4) supplementation in postmenopausal women for 3 years resulted in an increase, relative to placebo, in bone width and bone mineral content but not bone mineral density at the site of the femoral neck as measured by dual-energy x-ray absorptiometry. Another study in postmenopausal women by Knapen et al32 found that MK-7 supplementation decreased age-related decline in bone mineral concentration and bone mineral density at the lumbar spine and femoral neck but not at the total hip. Emaus et al43 also reported a lack of influence of vitamin K2 (MK-7) supplementation on bone loss in early menopausal women as measured by dual energy X-ray absorptiometry at the total hip, femoral neck, lumbar spine, and total body. Schurgers et al62 noted an effect, associated with menaquinones (eg, MK-7) only, of improved bone geometry contributing to bone strength during aging. The inconsistencies in the literature led Iwamoto et al41 to suggest that beneficial effects of vitamin K on the skeleton of postmenopausal women may be mediated, at least in part, by mechanisms other than changes in bone mineral density and bone turnover.
Other roles for vitamin K have been suggested by several studies that correlated a high concentration of MK-4 with the levels of sphingomyelin and sulfatides in the brain, indicating thatvitamin K may be involved in the biosynthesis of this important class of lipids.68–70 For these reasons, an adequate level of vitamin K is crucial for proper neurological development during the fetal and neonatal periods.15
Despite the many roles of vitamin K in the body, the most severe consequence of nutritional vitamin K deficiency is bleeding caused by relative inactivity of procoagulant proteins, a condition known as vitamin K–responsive hypoprothrombinemia. Spontaneous cases are rare and have usually been associated with various lipid malabsorption syndromes or antibiotic treatment–associated suppression of intestinal menaquinone-synthesizing bacteria.9,19
In infants aged 6 months or younger, vitamin K deficiency bleeding represents a significant public health problem worldwide.71 Early vitamin K deficiency bleeding, occurring in the first 24 hours of life, is rare and is typically seen in infants whose mothers have been prescribed drugs that interfere with vitamin K storage and metabolism in the newborn.71 Classic vitamin K deficiency bleeding, presenting between days 2 and 7 of life in as many as 1.7% of infants in the absence of the prophylaxis that is nearly universal in the United States and Canada,72,73 is related to low placental transfer of vitamin K. This leads to limited stores in the infant, low concentrations in breast milk, inadequate feeding or failure to establish early breastfeeding practices, an immature gastrointestinal tract, or lack of gastrointestinal flora in the newborn gut.71,72 Late vitamin K deficiency bleeding presents between day 8 and 6 months of life (peak incidence between weeks 3 and 8), with a prevalence of 4.4 to 7.2 cases per 100 000 births (much higher in low- and middle-income countries), and is often reported as idiopathic. However, there is growing evidence of an association with hepatobiliary dysfunction, which causes impaired secretion of bile salts and malabsorption of vitamin K, or other conditions that interfere with the synthesis and storage of clotting factors.9,19
Dietary restriction studies show that low intakes of dietary vitamin K1 can result in measurable decreases in the γ-carboxylation of both hepatic and bone Gla proteins after about 2 weeks, with a greater absolute change in the degree of osteocalcin carboxylation compared with prothrombin carboxylation.6 A decrease in levels of vitamin K–dependent coagulation proteins does not become clinically significant unless a deficient intake of vitamin K is prolonged for at least 1 month by a drastic reduction in sources of vitamin K, which may result from the use of antibiotics in parenterally fed patients. Such studies have demonstrated that intestinal bacteria provide a liver store of long-chain menaquinones, with slow turnover providing a considerable buffer that prevents any major effects on coagulation during short-term inadequate dietary intake of vitamin K. The liver contains a large pool of menaquinones, mostly long-chain forms in the range of 200–300 nmol (but with wide interindividual variations), which represents about 90% of the total liver vitamin K stores. Human bone tissue, however, lacks a similar reservoir and thus is more susceptible to vitamin K deficiency.6
Human requirements for vitamin K
The IOM (recently renamed the Health and Medicine Division) in 2001 found that data were insufficient to set an Estimated Average Requirement and thus calculate a Recommended Dietary Allowance for vitamin K, so Adequate Intake values for vitamin K1 were set on the basis of the highest median intake for each age group as reported by the National Health and Nutrition Examination Survey (NHANES) III.9 The Adequate Intake is the recommended average daily intake level based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people that are assumed to be adequate.
The IOM established an Adequate Intake for vitamin K1 of 120 µg/d for adult males (19–70+ years of age) and of 90 µg/d for adult females including during pregnancy and lactation. Using the IOM reference body weights of 70 kg for an adult male and 57 kg for an adult female, these intake levels would correspond to 1.7 and 1.6 µg/kg/d of vitamin K1 for males and females, respectively. Reference values for children were set at 2.0 µg/d for infants 0–6 months, 2.5 µg/d for infants 7–12 months, 30 µg/d for children 1–3 years, 55 µg/d for children 4–8 years, 60 µg/d for children 9–13 years, and 75 µg/d for adolescents of both sexes aged 14–18 years.9
The World Health Organization (WHO) and the Food and Agriculture Organization of the United Nations (FAO) jointly set Recommended Nutrient Intake levels for vitamin K1 of 65 µg/d for adult males and 55 µg/d for adult females, on the basis of 1 µg/kg/d, and scaled levels accordingly for other age groups.19
The Commission of the European Communities set a Recommended Daily Allowance for vitamin K of 75 µg/d.74
Biomarkers of vitamin K status in humans include plasma and serum concentrations of phylloquinone and menaquinones, urinary metabolites of vitamin K, factor VII activity, prothrombin times, urinary Gla residues, undercarboxylated hepatic prothrombin, undercarboxylated osteocalcin, and other uncarboxylated Gla proteins, although analysis of the physiological markers cannot differentiate between menaquinones and phylloquinone unless there is a direct comparison between the response to individual biomarkers and intakes of individual forms of vitamin K.9,14 Maintaining carboxylation of extrahepatic vitamin K–dependent proteins requires higher dietary vitamin K intake than that which is sufficient for the classic function of maintaining carboxylation of hepatic vitamin K–dependent coagulation proteins. This is because the presence of a considerable buffer of liver vitamin K stores prevents any major effects on coagulation during short-term dietary depletion in healthy individuals.6,12 In apparently healthy individuals without coagulation problems, the ratio of undercarboxylated to fully carboxylated osteocalcin can be as high as 45%,9 which has been interpreted as indicating a suboptimal vitamin K status with respect to bone health,14 as discussed above. Undercarboxylation of matrix Gla protein, a protein important for its role in reducing the risk of vascular calcification,33–37 would be suboptimal for cardiovascular health.14 Therefore, levels of circulating undercarboxylated extrahepatic vitamin K–dependent proteins such as osteocalcin and matrix Gla protein, which are known to respond to vitamin K supplementation, may be sensitive markers of vitamin K status.6,12,14
Exposure to vitamin K: intake of phylloquinone from the diet
To evaluate whether supplemental intake of vitamin K (as MK-7), in addition to dietary intake, might exceed the levels currently established as nutritional requirements, the Adequate Intake values can be compared with the most recent information on vitamin K1 intake in the United States, ie, the results of the NHANES 2011–2012 survey,75 summarized in Table 1.75 From this comparison, it can be seen that in the “all individuals” analysis, both males and females aged 20 years and above had mean dietary intakes of vitamin K1 that were greater than the Adequate Intake (116% and 134%, respectively). Among nonusers of vitamin K supplements, males aged 20–39 years and 40–59 years had intakes slightly below their Adequate Intake level (93% and 99%, respectively), but all adult females had a more-than-sufficient intake.
Mean daily intakes of vitamin K1 in the United States, 2011–201275
| Sex and age (y) . | Mean intake from food and beverages among all individualsa [µg (SE)] . | Percentage reporting vitamin K supplement useb . | Mean intake from food, beverages, and dietary supplements among supplement users [µg (SE)] . | Mean intake among nonusers of supplements [µg (SE)] . | ||||
|---|---|---|---|---|---|---|---|---|
| Sample size (no.) . | Food and beverages . | Sample size (no.) . | Food and beverages . | Supplement . | Food, beverages, and supplement . | Food and beverages . | ||
| Males and females | ||||||||
| 2–5 | 827 | 50.2 (1.96) | –c | –d | –d | –d | 50.2 (1.96) | |
| 6–11 | 1139 | 67.0 (3.48) | –c | –d | –d | –d | 67.0 (3.51) | |
| 12–19 | 1131 | 73.9 (5.22) | 3 | –d | –d | –d | 73.9 (5.05) | |
| Males | ||||||||
| 20–39 | 874 | 118.8 (6.99) | 12 | 81 | 171.9 (24.46) | 33.4 (3.55) | 205.4 (26.25) | 111.5 (7.14) |
| 40–59 | 752 | 129.0 (6.73) | 20 | 129 | 170.5 (24.84) | 31.3 (1.86) | 201.8 (24.40) | 118.6 (8.36) |
| ≥60 | 735 | 187.2e (61.68) | 29 | 179 | 115.0 (11.65) | 31.0 (1.65) | 146.0 (12.00) | 216.8e (86.16) |
| ≥20 | 2361 | 139.0 (16.42) | 19 | 389 | 150.7 (10.27) | 31.7 (1.10) | 182.4 (10.56) | 136.3 (19.88) |
| Females | ||||||||
| 20–39 | 756 | 110.5 (9.08) | 11 | –d | –d | –d | 112.9 (9.69) | |
| 40–59 | 806 | 128.2 (11.95) | 15 | 118 | 146.3 (40.33) | 28.9 (0.69) | 175.1 (40.23) | 125.0 (9.79) |
| ≥60 | 721 | 122.5 (8.83) | 29 | 180 | 134.3 (12.34) | 37.8 (3.05) | 172.1 (12.51) | 117.5 (10.37) |
| ≥20 | 2283 | 120.7 (7.23) | 17 | 364 | 129.4 (18.02) | 34.8 (1.99) | 164.2 (18.35) | 118.9 (6.32) |
| All | ||||||||
| ≥2 | 7741 | 113.7 (6.76) | 14 | 797 | 138.4 (9.07) | 33.1 (1.11) | 171.5 (9.34) | 109.6 (7.31) |
| Sex and age (y) . | Mean intake from food and beverages among all individualsa [µg (SE)] . | Percentage reporting vitamin K supplement useb . | Mean intake from food, beverages, and dietary supplements among supplement users [µg (SE)] . | Mean intake among nonusers of supplements [µg (SE)] . | ||||
|---|---|---|---|---|---|---|---|---|
| Sample size (no.) . | Food and beverages . | Sample size (no.) . | Food and beverages . | Supplement . | Food, beverages, and supplement . | Food and beverages . | ||
| Males and females | ||||||||
| 2–5 | 827 | 50.2 (1.96) | –c | –d | –d | –d | 50.2 (1.96) | |
| 6–11 | 1139 | 67.0 (3.48) | –c | –d | –d | –d | 67.0 (3.51) | |
| 12–19 | 1131 | 73.9 (5.22) | 3 | –d | –d | –d | 73.9 (5.05) | |
| Males | ||||||||
| 20–39 | 874 | 118.8 (6.99) | 12 | 81 | 171.9 (24.46) | 33.4 (3.55) | 205.4 (26.25) | 111.5 (7.14) |
| 40–59 | 752 | 129.0 (6.73) | 20 | 129 | 170.5 (24.84) | 31.3 (1.86) | 201.8 (24.40) | 118.6 (8.36) |
| ≥60 | 735 | 187.2e (61.68) | 29 | 179 | 115.0 (11.65) | 31.0 (1.65) | 146.0 (12.00) | 216.8e (86.16) |
| ≥20 | 2361 | 139.0 (16.42) | 19 | 389 | 150.7 (10.27) | 31.7 (1.10) | 182.4 (10.56) | 136.3 (19.88) |
| Females | ||||||||
| 20–39 | 756 | 110.5 (9.08) | 11 | –d | –d | –d | 112.9 (9.69) | |
| 40–59 | 806 | 128.2 (11.95) | 15 | 118 | 146.3 (40.33) | 28.9 (0.69) | 175.1 (40.23) | 125.0 (9.79) |
| ≥60 | 721 | 122.5 (8.83) | 29 | 180 | 134.3 (12.34) | 37.8 (3.05) | 172.1 (12.51) | 117.5 (10.37) |
| ≥20 | 2283 | 120.7 (7.23) | 17 | 364 | 129.4 (18.02) | 34.8 (1.99) | 164.2 (18.35) | 118.9 (6.32) |
| All | ||||||||
| ≥2 | 7741 | 113.7 (6.76) | 14 | 797 | 138.4 (9.07) | 33.1 (1.11) | 171.5 (9.34) | 109.6 (7.31) |
Abbreviation: SE, standard error.
aIncludes both supplement users and nonusers 2 years of age and over. Pregnant and/or lactating females, breastfed children, and individuals with an incomplete dietary supplement component of the 24-hour dietary recall were excluded.
bWeighted percentages of respondents who reported taking at least 1 multi- and /or single-nutrient supplement containing vitamin K.
cA nonzero value too small to report.
dEstimated mean not presented where sample size is less than 30 times the variance inflation factor.
eEstimated mean flagged as possibly less statistically reliable because of high relative standard error.
Mean daily intakes of vitamin K1 in the United States, 2011–201275
| Sex and age (y) . | Mean intake from food and beverages among all individualsa [µg (SE)] . | Percentage reporting vitamin K supplement useb . | Mean intake from food, beverages, and dietary supplements among supplement users [µg (SE)] . | Mean intake among nonusers of supplements [µg (SE)] . | ||||
|---|---|---|---|---|---|---|---|---|
| Sample size (no.) . | Food and beverages . | Sample size (no.) . | Food and beverages . | Supplement . | Food, beverages, and supplement . | Food and beverages . | ||
| Males and females | ||||||||
| 2–5 | 827 | 50.2 (1.96) | –c | –d | –d | –d | 50.2 (1.96) | |
| 6–11 | 1139 | 67.0 (3.48) | –c | –d | –d | –d | 67.0 (3.51) | |
| 12–19 | 1131 | 73.9 (5.22) | 3 | –d | –d | –d | 73.9 (5.05) | |
| Males | ||||||||
| 20–39 | 874 | 118.8 (6.99) | 12 | 81 | 171.9 (24.46) | 33.4 (3.55) | 205.4 (26.25) | 111.5 (7.14) |
| 40–59 | 752 | 129.0 (6.73) | 20 | 129 | 170.5 (24.84) | 31.3 (1.86) | 201.8 (24.40) | 118.6 (8.36) |
| ≥60 | 735 | 187.2e (61.68) | 29 | 179 | 115.0 (11.65) | 31.0 (1.65) | 146.0 (12.00) | 216.8e (86.16) |
| ≥20 | 2361 | 139.0 (16.42) | 19 | 389 | 150.7 (10.27) | 31.7 (1.10) | 182.4 (10.56) | 136.3 (19.88) |
| Females | ||||||||
| 20–39 | 756 | 110.5 (9.08) | 11 | –d | –d | –d | 112.9 (9.69) | |
| 40–59 | 806 | 128.2 (11.95) | 15 | 118 | 146.3 (40.33) | 28.9 (0.69) | 175.1 (40.23) | 125.0 (9.79) |
| ≥60 | 721 | 122.5 (8.83) | 29 | 180 | 134.3 (12.34) | 37.8 (3.05) | 172.1 (12.51) | 117.5 (10.37) |
| ≥20 | 2283 | 120.7 (7.23) | 17 | 364 | 129.4 (18.02) | 34.8 (1.99) | 164.2 (18.35) | 118.9 (6.32) |
| All | ||||||||
| ≥2 | 7741 | 113.7 (6.76) | 14 | 797 | 138.4 (9.07) | 33.1 (1.11) | 171.5 (9.34) | 109.6 (7.31) |
| Sex and age (y) . | Mean intake from food and beverages among all individualsa [µg (SE)] . | Percentage reporting vitamin K supplement useb . | Mean intake from food, beverages, and dietary supplements among supplement users [µg (SE)] . | Mean intake among nonusers of supplements [µg (SE)] . | ||||
|---|---|---|---|---|---|---|---|---|
| Sample size (no.) . | Food and beverages . | Sample size (no.) . | Food and beverages . | Supplement . | Food, beverages, and supplement . | Food and beverages . | ||
| Males and females | ||||||||
| 2–5 | 827 | 50.2 (1.96) | –c | –d | –d | –d | 50.2 (1.96) | |
| 6–11 | 1139 | 67.0 (3.48) | –c | –d | –d | –d | 67.0 (3.51) | |
| 12–19 | 1131 | 73.9 (5.22) | 3 | –d | –d | –d | 73.9 (5.05) | |
| Males | ||||||||
| 20–39 | 874 | 118.8 (6.99) | 12 | 81 | 171.9 (24.46) | 33.4 (3.55) | 205.4 (26.25) | 111.5 (7.14) |
| 40–59 | 752 | 129.0 (6.73) | 20 | 129 | 170.5 (24.84) | 31.3 (1.86) | 201.8 (24.40) | 118.6 (8.36) |
| ≥60 | 735 | 187.2e (61.68) | 29 | 179 | 115.0 (11.65) | 31.0 (1.65) | 146.0 (12.00) | 216.8e (86.16) |
| ≥20 | 2361 | 139.0 (16.42) | 19 | 389 | 150.7 (10.27) | 31.7 (1.10) | 182.4 (10.56) | 136.3 (19.88) |
| Females | ||||||||
| 20–39 | 756 | 110.5 (9.08) | 11 | –d | –d | –d | 112.9 (9.69) | |
| 40–59 | 806 | 128.2 (11.95) | 15 | 118 | 146.3 (40.33) | 28.9 (0.69) | 175.1 (40.23) | 125.0 (9.79) |
| ≥60 | 721 | 122.5 (8.83) | 29 | 180 | 134.3 (12.34) | 37.8 (3.05) | 172.1 (12.51) | 117.5 (10.37) |
| ≥20 | 2283 | 120.7 (7.23) | 17 | 364 | 129.4 (18.02) | 34.8 (1.99) | 164.2 (18.35) | 118.9 (6.32) |
| All | ||||||||
| ≥2 | 7741 | 113.7 (6.76) | 14 | 797 | 138.4 (9.07) | 33.1 (1.11) | 171.5 (9.34) | 109.6 (7.31) |
Abbreviation: SE, standard error.
aIncludes both supplement users and nonusers 2 years of age and over. Pregnant and/or lactating females, breastfed children, and individuals with an incomplete dietary supplement component of the 24-hour dietary recall were excluded.
bWeighted percentages of respondents who reported taking at least 1 multi- and /or single-nutrient supplement containing vitamin K.
cA nonzero value too small to report.
dEstimated mean not presented where sample size is less than 30 times the variance inflation factor.
eEstimated mean flagged as possibly less statistically reliable because of high relative standard error.
To illustrate change over time in vitamin K1 dietary intake, current intake data can be compared with the findings of the NHANES III (1988–1994) survey. Intake of vitamin K1 from food (not including supplements) was reported in NHANES III as the following mean (standard error) values for males in the age groups 19–30, 31–50, 51–70, and 71+ years: 105.8 (12.6), 125.4 (11.4), 120.0 (8.5), and 97.8 (8.1) µg/d, respectively, with a 5th percentile range of 44–63 µg/d and a 95th percentile range of 181–223 µg/d. For adult females, the mean (standard error) values were 98.0 (14.6), 99.6 (3.3), 97.2 (4.4), and 93.8 (4.3) µg/d, respectively, for the same age groups, with a 5th percentile range of 32–38 µg/d and a 95th percentile range of 204–217 µg/d.9 Thus, in the years between the NHANES III (1988–1994) and NHANES 2011–2012 surveys, on the basis of data from all individuals, the adult mean intake of vitamin K1 from food appears to have increased by approximately 20%. However, since the NHANES III (1988–1994) data were compiled, food composition databases for vitamin K have improved dramatically, so it is plausible that the observed increase in intakes in the NHANES 2011–2012 surveys could reflect, at least in part, the improved methods of quantifying vitamin K in food sources. In addition, given the limited availability of databases for menaquinone intakes and the results of studies showing that long-chain menaquinones are more abundant in the food supply than previously thought,76 it is plausible that the current reported intake may be an underestimate.
In a review of 11 other studies of vitamin K intake in the United States and other countries, Booth and Suttie77 found that young adults have a phylloquinone intake of approximately 80 µg/d and older adults (>55 y) an intake of approximately 150 µg/d, but the vitamin K mean intake estimates ranged from 61 to 210 µg/d.
Shea et al78 examined racial and ethnic differences in serum vitamin K1 levels and found that 25% of individuals (N = 704) had levels of less than 0.1 nmol/L, which is the lower limit of detection for this assay. The prevalence of low serum vitamin K1 was 4% in Chinese Americans, compared with 24% in white Americans, 29% in African Americans, and 33% in Hispanic Americans.
Exposure to vitamin K: intake of menaquinones from the diet
Reported natural levels of menaquinones, particularly MK-7, in a variety of foods are listed in Table 2.21,79–82 The richest dietary sources of long-chain menaquinones are foods fermented by bacteria (not by molds or yeasts), typically represented in Western diets by dairy products such as cheeses (MK-8, MK-9) and in the Japanese diet by natto (MK-7). Apart from animal livers, meat and fish products generally are low in long-chain menaquinones and are likely of little importance as dietary sources of vitamin K2.12 As seen in Table 2, the most commonly encountered menaquinones in the human diet have a side chain with 4, 6, 7, 8, 9, or 10 isoprene residues.
| Food . | Menaquinones and vitamin K1 levels (per 100 g) . |
|---|---|
| Natto | ND to 2.0 µg MK-4, 7.5 µg MK-5, 13.8 µg MK-6, 939 – 998 µg MK-7, 84.1 µg MK-8, 34.7 µg K1 |
| Jarlsberg and Emmental cheeses | 20 – 65 µg MK-7 |
| Hard cheeses (eg, cheddar) | Up to 10.2 µg MK-4, 1.5 µg MK-5, up to 3.0 µg MK-6, up to 2.3 µg MK-7, 16.9 µg MK-8, 51.1 µg MK-9, 10.4 µg K1 |
| Semi-firm cheeses | 1.0 – 3.5 µg MK-6, ND to 2.1 µg MK-7, 2.5 – 7.3 µg MK-8, 10.0 – 32.1 µg MK-9, ND to 13.8 µg MK-10 |
| Soft cheeses | 3.7 µg MK-4, 0.3 µg MK-5, 0.4 – 2.6 µg MK-6, ND to 1.7 µg MK-7, 2.1 – 14.0 µg MK-8, 6.6 – 94.0 µg MK-9, ND to 5.7 µg MK-10, 2.6 µg K1 |
| Curd cheese | 0.4 µg MK-4, 0.1 µg MK-5, 0.2 µg MK-6, 0.3 µg MK-7, 5.1 µg MK-8, 18.7 µg MK-9, 0.3 µg K1 |
| Chicken, barbequed | 22.1 µg MK-4, 2.0 µg K1, 0.9 µg dihydrophylloquinone |
| Chicken liver, pan fried | 12.6 µg MK-4 |
| Meat franks, regular fat, cooked | 9.8 µg MK-4, 2.5 µg K1 |
| Salami | 9.0 µg MK-4, 2.3 µg K1 |
| Ground beef, medium fat, broiled | 7.2 µg MK-4, 1.4 µg K1 |
| Calf liver, pan fried | 6.0 µg MK-4, 2.1 µg K1 |
| Hot dog, beef, regular fat, cooked | 5.7 µg MK-4, 4.3 µg K1 |
| Bacon, pan fried | 5.6 µg MK-4 |
| Ground beef, high fat, broiled | 5.1 µg MK-4, 2.1 µg K1 |
| Ham, roasted or pan broiled | 5.1 µg MK-4 |
| Sauerkraut | 0.4 µg MK-4, 0.8 µg MK-5, 1.5 µg MK-6, 0.2 µg MK-7, 0.8 µg MK-8, 1.1 µg MK-9, 25 µg K1 |
| Egg, cooked | 4.0 µg MK-4 |
| Breakfast sausage | 3.0 µg MK-4, 3.5 µg K1, 11.7 µg dihydrophylloquinone |
| Pork steak | 2.1 µg MK-4, 0.5 µg MK-7, 1.1 µg MK-8, 0.3 µg K1 |
| Buttermilk, whole fat | 0.2 µg MK-4, 0.1 µg MK-5, 0.1 µg MK-6, 0.1 µg MK-7, 0.6 µg MK-8, 1.4 µg MK-9 |
| Beef liver, braised | 1.9 µg MK-4, 3.3 µg K1 |
| Ground beef, low fat, broiled | 1.7 µg MK-4, 1.2 µg K1 |
| Eel | 1.7 µg MK-4, 0.1 µg MK-6. 0.4 µg MK-7, 0.3 µg K1 |
| Milk, whole | 0.8 µg MK-4, 0.1 µg MK-5, ND to 2.0 µg MK-7, 0.5 µg K1 |
| Plaice | 0.2 µg MK-4, 0.3 µg MK-6, 0.1 µg MK-7, 1.6 µg MK-8 |
| Salmon, sockeye, cooked | 0.4 µg MK-4 |
| Shrimp, cooked | 0.4 µg MK-4 |
| Yogurt | Up to 0.4 µg MK-7 |
| Food . | Menaquinones and vitamin K1 levels (per 100 g) . |
|---|---|
| Natto | ND to 2.0 µg MK-4, 7.5 µg MK-5, 13.8 µg MK-6, 939 – 998 µg MK-7, 84.1 µg MK-8, 34.7 µg K1 |
| Jarlsberg and Emmental cheeses | 20 – 65 µg MK-7 |
| Hard cheeses (eg, cheddar) | Up to 10.2 µg MK-4, 1.5 µg MK-5, up to 3.0 µg MK-6, up to 2.3 µg MK-7, 16.9 µg MK-8, 51.1 µg MK-9, 10.4 µg K1 |
| Semi-firm cheeses | 1.0 – 3.5 µg MK-6, ND to 2.1 µg MK-7, 2.5 – 7.3 µg MK-8, 10.0 – 32.1 µg MK-9, ND to 13.8 µg MK-10 |
| Soft cheeses | 3.7 µg MK-4, 0.3 µg MK-5, 0.4 – 2.6 µg MK-6, ND to 1.7 µg MK-7, 2.1 – 14.0 µg MK-8, 6.6 – 94.0 µg MK-9, ND to 5.7 µg MK-10, 2.6 µg K1 |
| Curd cheese | 0.4 µg MK-4, 0.1 µg MK-5, 0.2 µg MK-6, 0.3 µg MK-7, 5.1 µg MK-8, 18.7 µg MK-9, 0.3 µg K1 |
| Chicken, barbequed | 22.1 µg MK-4, 2.0 µg K1, 0.9 µg dihydrophylloquinone |
| Chicken liver, pan fried | 12.6 µg MK-4 |
| Meat franks, regular fat, cooked | 9.8 µg MK-4, 2.5 µg K1 |
| Salami | 9.0 µg MK-4, 2.3 µg K1 |
| Ground beef, medium fat, broiled | 7.2 µg MK-4, 1.4 µg K1 |
| Calf liver, pan fried | 6.0 µg MK-4, 2.1 µg K1 |
| Hot dog, beef, regular fat, cooked | 5.7 µg MK-4, 4.3 µg K1 |
| Bacon, pan fried | 5.6 µg MK-4 |
| Ground beef, high fat, broiled | 5.1 µg MK-4, 2.1 µg K1 |
| Ham, roasted or pan broiled | 5.1 µg MK-4 |
| Sauerkraut | 0.4 µg MK-4, 0.8 µg MK-5, 1.5 µg MK-6, 0.2 µg MK-7, 0.8 µg MK-8, 1.1 µg MK-9, 25 µg K1 |
| Egg, cooked | 4.0 µg MK-4 |
| Breakfast sausage | 3.0 µg MK-4, 3.5 µg K1, 11.7 µg dihydrophylloquinone |
| Pork steak | 2.1 µg MK-4, 0.5 µg MK-7, 1.1 µg MK-8, 0.3 µg K1 |
| Buttermilk, whole fat | 0.2 µg MK-4, 0.1 µg MK-5, 0.1 µg MK-6, 0.1 µg MK-7, 0.6 µg MK-8, 1.4 µg MK-9 |
| Beef liver, braised | 1.9 µg MK-4, 3.3 µg K1 |
| Ground beef, low fat, broiled | 1.7 µg MK-4, 1.2 µg K1 |
| Eel | 1.7 µg MK-4, 0.1 µg MK-6. 0.4 µg MK-7, 0.3 µg K1 |
| Milk, whole | 0.8 µg MK-4, 0.1 µg MK-5, ND to 2.0 µg MK-7, 0.5 µg K1 |
| Plaice | 0.2 µg MK-4, 0.3 µg MK-6, 0.1 µg MK-7, 1.6 µg MK-8 |
| Salmon, sockeye, cooked | 0.4 µg MK-4 |
| Shrimp, cooked | 0.4 µg MK-4 |
| Yogurt | Up to 0.4 µg MK-7 |
Abbreviation: ND, not determined.
| Food . | Menaquinones and vitamin K1 levels (per 100 g) . |
|---|---|
| Natto | ND to 2.0 µg MK-4, 7.5 µg MK-5, 13.8 µg MK-6, 939 – 998 µg MK-7, 84.1 µg MK-8, 34.7 µg K1 |
| Jarlsberg and Emmental cheeses | 20 – 65 µg MK-7 |
| Hard cheeses (eg, cheddar) | Up to 10.2 µg MK-4, 1.5 µg MK-5, up to 3.0 µg MK-6, up to 2.3 µg MK-7, 16.9 µg MK-8, 51.1 µg MK-9, 10.4 µg K1 |
| Semi-firm cheeses | 1.0 – 3.5 µg MK-6, ND to 2.1 µg MK-7, 2.5 – 7.3 µg MK-8, 10.0 – 32.1 µg MK-9, ND to 13.8 µg MK-10 |
| Soft cheeses | 3.7 µg MK-4, 0.3 µg MK-5, 0.4 – 2.6 µg MK-6, ND to 1.7 µg MK-7, 2.1 – 14.0 µg MK-8, 6.6 – 94.0 µg MK-9, ND to 5.7 µg MK-10, 2.6 µg K1 |
| Curd cheese | 0.4 µg MK-4, 0.1 µg MK-5, 0.2 µg MK-6, 0.3 µg MK-7, 5.1 µg MK-8, 18.7 µg MK-9, 0.3 µg K1 |
| Chicken, barbequed | 22.1 µg MK-4, 2.0 µg K1, 0.9 µg dihydrophylloquinone |
| Chicken liver, pan fried | 12.6 µg MK-4 |
| Meat franks, regular fat, cooked | 9.8 µg MK-4, 2.5 µg K1 |
| Salami | 9.0 µg MK-4, 2.3 µg K1 |
| Ground beef, medium fat, broiled | 7.2 µg MK-4, 1.4 µg K1 |
| Calf liver, pan fried | 6.0 µg MK-4, 2.1 µg K1 |
| Hot dog, beef, regular fat, cooked | 5.7 µg MK-4, 4.3 µg K1 |
| Bacon, pan fried | 5.6 µg MK-4 |
| Ground beef, high fat, broiled | 5.1 µg MK-4, 2.1 µg K1 |
| Ham, roasted or pan broiled | 5.1 µg MK-4 |
| Sauerkraut | 0.4 µg MK-4, 0.8 µg MK-5, 1.5 µg MK-6, 0.2 µg MK-7, 0.8 µg MK-8, 1.1 µg MK-9, 25 µg K1 |
| Egg, cooked | 4.0 µg MK-4 |
| Breakfast sausage | 3.0 µg MK-4, 3.5 µg K1, 11.7 µg dihydrophylloquinone |
| Pork steak | 2.1 µg MK-4, 0.5 µg MK-7, 1.1 µg MK-8, 0.3 µg K1 |
| Buttermilk, whole fat | 0.2 µg MK-4, 0.1 µg MK-5, 0.1 µg MK-6, 0.1 µg MK-7, 0.6 µg MK-8, 1.4 µg MK-9 |
| Beef liver, braised | 1.9 µg MK-4, 3.3 µg K1 |
| Ground beef, low fat, broiled | 1.7 µg MK-4, 1.2 µg K1 |
| Eel | 1.7 µg MK-4, 0.1 µg MK-6. 0.4 µg MK-7, 0.3 µg K1 |
| Milk, whole | 0.8 µg MK-4, 0.1 µg MK-5, ND to 2.0 µg MK-7, 0.5 µg K1 |
| Plaice | 0.2 µg MK-4, 0.3 µg MK-6, 0.1 µg MK-7, 1.6 µg MK-8 |
| Salmon, sockeye, cooked | 0.4 µg MK-4 |
| Shrimp, cooked | 0.4 µg MK-4 |
| Yogurt | Up to 0.4 µg MK-7 |
| Food . | Menaquinones and vitamin K1 levels (per 100 g) . |
|---|---|
| Natto | ND to 2.0 µg MK-4, 7.5 µg MK-5, 13.8 µg MK-6, 939 – 998 µg MK-7, 84.1 µg MK-8, 34.7 µg K1 |
| Jarlsberg and Emmental cheeses | 20 – 65 µg MK-7 |
| Hard cheeses (eg, cheddar) | Up to 10.2 µg MK-4, 1.5 µg MK-5, up to 3.0 µg MK-6, up to 2.3 µg MK-7, 16.9 µg MK-8, 51.1 µg MK-9, 10.4 µg K1 |
| Semi-firm cheeses | 1.0 – 3.5 µg MK-6, ND to 2.1 µg MK-7, 2.5 – 7.3 µg MK-8, 10.0 – 32.1 µg MK-9, ND to 13.8 µg MK-10 |
| Soft cheeses | 3.7 µg MK-4, 0.3 µg MK-5, 0.4 – 2.6 µg MK-6, ND to 1.7 µg MK-7, 2.1 – 14.0 µg MK-8, 6.6 – 94.0 µg MK-9, ND to 5.7 µg MK-10, 2.6 µg K1 |
| Curd cheese | 0.4 µg MK-4, 0.1 µg MK-5, 0.2 µg MK-6, 0.3 µg MK-7, 5.1 µg MK-8, 18.7 µg MK-9, 0.3 µg K1 |
| Chicken, barbequed | 22.1 µg MK-4, 2.0 µg K1, 0.9 µg dihydrophylloquinone |
| Chicken liver, pan fried | 12.6 µg MK-4 |
| Meat franks, regular fat, cooked | 9.8 µg MK-4, 2.5 µg K1 |
| Salami | 9.0 µg MK-4, 2.3 µg K1 |
| Ground beef, medium fat, broiled | 7.2 µg MK-4, 1.4 µg K1 |
| Calf liver, pan fried | 6.0 µg MK-4, 2.1 µg K1 |
| Hot dog, beef, regular fat, cooked | 5.7 µg MK-4, 4.3 µg K1 |
| Bacon, pan fried | 5.6 µg MK-4 |
| Ground beef, high fat, broiled | 5.1 µg MK-4, 2.1 µg K1 |
| Ham, roasted or pan broiled | 5.1 µg MK-4 |
| Sauerkraut | 0.4 µg MK-4, 0.8 µg MK-5, 1.5 µg MK-6, 0.2 µg MK-7, 0.8 µg MK-8, 1.1 µg MK-9, 25 µg K1 |
| Egg, cooked | 4.0 µg MK-4 |
| Breakfast sausage | 3.0 µg MK-4, 3.5 µg K1, 11.7 µg dihydrophylloquinone |
| Pork steak | 2.1 µg MK-4, 0.5 µg MK-7, 1.1 µg MK-8, 0.3 µg K1 |
| Buttermilk, whole fat | 0.2 µg MK-4, 0.1 µg MK-5, 0.1 µg MK-6, 0.1 µg MK-7, 0.6 µg MK-8, 1.4 µg MK-9 |
| Beef liver, braised | 1.9 µg MK-4, 3.3 µg K1 |
| Ground beef, low fat, broiled | 1.7 µg MK-4, 1.2 µg K1 |
| Eel | 1.7 µg MK-4, 0.1 µg MK-6. 0.4 µg MK-7, 0.3 µg K1 |
| Milk, whole | 0.8 µg MK-4, 0.1 µg MK-5, ND to 2.0 µg MK-7, 0.5 µg K1 |
| Plaice | 0.2 µg MK-4, 0.3 µg MK-6, 0.1 µg MK-7, 1.6 µg MK-8 |
| Salmon, sockeye, cooked | 0.4 µg MK-4 |
| Shrimp, cooked | 0.4 µg MK-4 |
| Yogurt | Up to 0.4 µg MK-7 |
Abbreviation: ND, not determined.
In contrast to dietary intakes of phylloquinone, intakes of menaquinone have not been well studied. In the Netherlands, estimates derived from food frequency questionnaires (N = 5435) were used to calculate intake levels. The overall mean daily intake of longer-chain menaquinones (MK-5 through MK-10) was 6.6, 16.9, 25.1, and 36.5 µg/d for quartiles 1 through 4, respectively, and of MK-4 was 3.7, 5.7, 7.6, and 9.9 µg/d, compared with vitamin K1 intakes of 124, 213, 278, and 375 µg/d.83
Menaquinones produced by the bacterial flora in the intestine are absorbed to some extent and contribute to the daily vitamin K requirement.6 The human liver contains about 10 times as much menaquinones as phylloquinone.9 Of the 2 major genera of anaerobic bacteria in the intestinal flora, Bacteroides and Bifidobacterium, only Bacteroides biosynthesizes menaquinones, mostly MK-10 and MK-11, along with minor amounts of MK-7, MK-8, MK-9, and MK-12.6
Sakano et al16 quantified menaquinones in human feces from Japanese individuals aged 24–41 years as follows: K1, 1857 ng/g dry weight; MK-4, 163 ng/g; MK-5, 223 ng/g; MK-6, 830 ng/g; MK-7, 787 ng/g; MK-8, 200 ng/g; MK-9, 787 ng/g; MK-10, 7553 ng/g; MK-11, 3613 ng/g; MK-12, 3370 ng/g; MK-13, 3623 ng/g; MK-14, 570 ng/g; and MK-15, 60 ng/g.
The same research team found the following levels in human plasma samples: K1, 1.16 ng/mL; MK-4, 0.3 ng/mL; MK-5, 0.08 ng/mL; MK-6, 0.21 ng/mL; MK-7, 0.37 ng/mL; and MK-8, 0.20 ng/mL.84 It cannot be determined from these 2 studies which proportion of the MK-7 in human plasma or in human feces originated from the diet vs the intestinal microflora, since MK-7 is produced from both sources.
Exposure to vitamin K: intake from dietary supplements
The NHANES 2011–201275 results for vitamin K1 intake, summarized in Table 1, show that the percentages of the US population reporting use of vitamin K supplements were negligible in children and very low in adolescents, about 19% in adult males, and about 17% in adult females. Regarding food and beverages, from the “all individuals” portion of the analysis, adult males had a mean intake of 139 µg/d and adult females 121 µg/d (percentile intake values were not reported). Among adult vitamin K supplement users, the mean intake from food and beverages was 151 µg/d in males and 129 µg/d in females, and the additional intake from supplements was 32 µg/d in males and 35 µg/d in females. Thus, supplemental vitamin K intake in combination with dietary intake resulted in an intake well above the Adequate Intake (152% in males and 182% in females).
With regard to change over time, the NHANES III (1988–1994)9 data show the mean intake of vitamin K1 from food plus supplements among all individuals to be 95.3 µg/d, compared with 93.7 µg/d from food alone. The differences in vitamin K1 intake between food plus supplements and food alone in each adult age and sex group were similar, indicating a very small contribution, at that time, of approximately 2 µg/d of vitamin K1 from supplements, compared with the current intake of approximately 30 µg/d from supplements.
Menaquinone-7 is an ingredient in currently marketed dietary supplements. The Dietary Supplement Label Database85 lists more than 200 products containing MK-7, the majority of which have a manufacturer’s suggested MK-7 dose of 50 µg/d. The lowest recommended intake is 5 µg/d, and the highest, 600 µg/d. Supplement Facts labeling of the Percent Daily Value (% DV), against which a product’s content of vitamin K is reported, is currently based on a Reference Daily Intake of 80 µg/d, which was recently revised to the Acceptable Daily Intake of 120 µg/d.86
In Canada, dietary supplements are regulated as a subset of drugs in accordance with the Natural Health Products Regulations. Health Canada’s Natural Health Products Ingredients Database87 lists vitamin K1, vitamin K2, MK-4, MK-6, and MK-7 as medicinal ingredients of natural health products. The Licensed Natural Health Products Database88 lists more than 500 marketed natural health products with vitamin K2 (the specific menaquinone is not indicated, but in some cases the brand name suggests MK-7). There are more than 1000 marketed natural health products containing vitamin K1. For both menaquinones and phylloquinone, products providing doses of up to a maximum of 120 µg/d are available. A prescription is required for any vitamers other than K1 or K2 and for any oral forms of K1 or K2 providing more than 120 µg/d.89
The European Food Safety Authority (EFSA), which assessed vitamin K2 added for nutritional purposes to foodstuffs, noted that the proposed level of use of 10 µg per serving for various foods would result in estimated mean intakes of MK-7, based on conservative assumptions, that ranged from 36 µg/d to 54 µg/d by female adults and male teenagers, respectively. The highest 97.5th percentile intake was in children and amounted to 5.4 µg/kg/d.24
Safety of vitamin K: literature reviews and a prospective cohort analysis
A search of the literature by the IOM revealed no evidence of toxicity associated with either the natural menaquinones or phylloquinone. Given the lack of adverse effects in humans or animals consuming high doses of vitamin K, the IOM was unable to derive a Tolerable Upper Intake Level.9 Therefore, supplemental plus dietary vitamin K1 intake levels of 152% and 182% of the Adequate Intake for adult males and females, respectively, cannot be interpreted as unsafe.
In its opinion on the Tolerable Upper Intake Level of vitamin K, the European Commission’s Scientific Committee on Food90 found no evidence of adverse effects associated with supplemental intake of vitamin K1 at up to 10 mg/d for limited periods of time. This conclusion was also supported by experimental animal studies in which no adverse effects were observed after daily administration of extremely high doses (2000 mg/kg) for 30 days.
The United Kingdom’s Expert Group on Vitamins and Minerals (UK EVM) also concluded that the available evidence from human and animal studies was insufficient to set a Safe Upper Level for vitamin K.10 However, a Guidance Level of 1 mg/d (or 0.017 mg/kg/d in a 60-kg adult) was established for supplemental intake of vitamin K1. This was based on limited human supplementation studies, which showed that doses of up to 10 mg/d of vitamin K1 for 1 month were not associated with adverse effects, and was calculated by applying an Uncertainty Factor of 10 for interindividual variation because of the very limited human database.
Hathcock91 also reviewed the safety of vitamin K and concluded that the potential for toxicity is extremely low, although the data were insufficient to establish how low. He considered the UK EVM decision to apply an Uncertainty Factor of 10 unnecessarily cautious in view of the absence of reports of adverse effects at intakes of up to 30 mg/d or more. Applying the Observed Safe Level method, he identified the Upper Level for Supplements for vitamin K as 10 mg/d, on the basis of the same clinical data identified by the UK EVM but using an Uncertainty Factor of 1 instead of 10.91 He noted that this Upper Level for Supplements does not apply to persons taking anticoagulant drugs, because such drugs interact strongly with vitamin K.
The WHO/FAO19 concluded that oral natural K vitamins seem to be free of toxic side effects, an observation borne out by clinical administration of doses of 10–20 mg/d or more.
The EFSA Panel on Dietetic Products, Nutrition and Allergies conducted a safety assessment of vitamin K2 (principally MK-7 with a small amount of MK-6) added for nutritional purposes to foodstuffs intended for the general population.24 The panel reviewed the available literature up to the end of 2008, including unpublished clinical studies provided by the petitioner, and concluded there were no adverse effects of MK-7 on blood coagulation at doses of up to 6 µg/kg/d in adults and 1.5 µg/kg/d in children.
With regard to reproductive and developmental toxicity, the EFSA review found no adverse effects of MK-4 in healthy newborn infants (n = 81 given 2 mg twice in first week of life, then n = 35 with maternal supplementation at 15 mg/d for 2 weeks vs n = 46 with no maternal supplementation).24 In rodent studies with MK-4, no significant differences between treatment and control groups were observed in total number of implants, percentage of resorptions, number of dead or live fetuses, mean body weight, and type and number of anomalies in mice or rat fetuses, irrespective of the route of administration, food and water consumption, or body weight gain of either sex. No compound-related effects on reproductive or developmental parameters were observed, including number of corpora lutea, number of implantations, implantation ratio, percentage of resorptions and viable fetuses, fetal and placental weights, and incidence of internal and external malformations. One exception was the observation of an increased incidence of nonossified forelimbs in mouse fetuses obtained from mid- and high-dose groups compared with the control group, which may indicate some delay in development.
Reviewing genotoxicity, mutagenicity, and carcinogenicity, the EFSA noted that positive results were reported in a nonspecific DNA repair test following incubation of vitamin K2 (vitamer not specified) with Escherichia coli, but when subjected to a mammalian test system, MK-4 produced no significant increase in the incidence of single-strand DNA breaks.24 The EFSA conclusions agreed with those of the review published in 2000 by the International Agency for Research on Cancer. The EFSA also referred to the International Agency for Research on Cancer’s placement of vitamin K substances into Group 3 (ie, not classifiable as to their carcinogenicity in humans), which was based on inadequate evidence for carcinogenicity in humans and experimental animals. The EFSA noted there was no evidence of any putative preneoplastic or hyperplastic lesion in several subchronic (13 weeks) and 2 long-term (1 year) toxicity studies in rats and dogs given oral doses of MK-4.
On the basis of the results of a 1-year study in rats to investigate the association between MK-4 intake and a decrease in prothrombin time, using daily oral doses of MK-4 at 0, 20, 100, and 500 mg/kg of body weight, the EFSA derived 20 mg/kg/d as the LOAEL.24 The EFSA calculated the Margin of Safety from the highest 97.5th percentile intake estimate for children (5.4 µg/kg/d) and the LOAEL (20 mg/kg) from the rat study to be 3700. The conservative estimate of an intake of 5.4 µg/kg/d is also 3.6-fold higher than the dose shown not to affect blood clotting parameters in a study in children and is just below the 6 µg/kg/d shown not to affect blood clotting in adults. The EFSA concluded that the use of MK-7 in foods for the general population (including food supplements) and in foods for particular nutritional uses, other than baby foods and infant formula, at the proposed use levels (no serving >10 µg) does not pose a safety concern.
Ronden et al92 demonstrated that very high doses of vitamin K in rats (250 mg/kg/d) did not affect either the blood coagulation characteristics or the blood platelet aggregation rate. It is important to note that a decrease in prothrombin time cannot be expected from high doses of vitamin K because, in the general human population, all Gla-containing clotting factors are fully carboxylated at levels of vitamin K intake close to the Recommended Dietary Allowance.33
More recently, Heinonen et al93 conducted a scientific literature review for the EFSA in preparation for the establishment of a Dietary Reference Value for vitamin K. They found a favorable association between both K1 and K2 intake and bone health markers, no association between K1 or K2 intake and prostate cancer, a positive association between both K1 and K2 intake and a reduced risk of coronary heart disease and between both K1 and K2 intake and a reduced risk of type 2 diabetes, and an inverse relationship between K2 intake and all-cause mortality in long-term follow-up.
While not direct measures of vitamin K safety, the conclusions of the reviews by EFSA and other reputable agencies are supportive of a favorable benefit to risk ratio.9,10,19,24,90,94
It should also be noted that GRAS Associates LLC, Bonita Springs, Florida, on behalf of NattoPharma ASA, Oslo, Norway, submitted to the US Food and Drug Administration (FDA) a self-affirmed safety assessment as a GRAS (Generally Recognized as Safe) notice (no. GRN 000245) for MK-7 (with a minor amount of MK-6) from B subtilis natto fermentation of soy protein extracted with ethanol and formulated as a corn oil suspension.23 However, they withdrew it before the FDA could review it, and thus the agency made no determination on it.95
Juanola-Falgarona et al,96 in a prospective cohort analysis of 7216 participants at high cardiovascular disease risk, found that a higher baseline dietary vitamin K1 intake was associated with a significantly lower risk of cancer and all-cause mortality. Individuals who increased their intake of vitamin K1 or K2 during follow-up (median, 4.8 years) also had a significantly lower risk of cancer and all-cause mortality than those who decreased or did not change their intake. While an increase in dietary vitamin K1 intake was associated with a significantly lower cardiovascular mortality risk, no association with cardiovascular mortality risk was observed with an increase in dietary vitamin K2 intake.
Functional relationships between various forms of vitamin K
The functional relationship between the various menaquinones and phylloquinone, which is complex and involves both direct roles and interconversion, is an important aspect of safety. As noted previously, phylloquinone is the major dietary form of vitamin K, with much smaller contributions coming from the menaquinones.83 However, in humans, vitamin K1 constitutes only 10% of hepatic vitamin K stores, which normally consist of about 90% menaquinones (mainly MK-6 through MK-13).24 Menaquinone-4 is biosynthesized primarily by the human body and is ubiquitous in extrahepatic tissues, with significant amounts present in the salivary glands, kidneys, pancreas, and brain.24,97
Most of the natural vitamin K homologues can be converted to MK-4 in vivo. Urinary menadione excretion increases greatly after oral intake of phylloquinone, MK-4, and MK-7. This effect is apparent within 1–2 hours and peaks at about 3 hours after intake. Amounts of menadione glucuronides and sulfates excreted in 24 hours after vitamin K intake range, on a molar basis, from 1% to 5% of the administered dose. This indicates that about 5% to 25% of the ingested K vitamins are catabolized to menadione in healthy male volunteers.98 In rat experiments, Hirota et al99 showed that, during the course of absorption, cleavage of the phytyl side chain of vitamin K1 or the isoprenoid side chain of menaquinones within the intestinal enterocytes leads to the production of menadione, which is converted via a hydroquinone intermediate to MK-4 in peripheral tissues. Nakagawa et al97 identified a prenyltransferase involved in MK-4 biosynthesis in human osteoblast-like cells that might catalyze prenylation of menadione to MK-4, suggesting that the same interconversion seen in rats may also be present in humans.
A further complication in comparing the structure–function relationships of vitamin K homologs is related to the mechanism of action of those homologs. Vitamin K–dependent carboxylase is a bifunctional enzyme that catalyzes the oxygenation of vitamin K hydroquinone to vitamin K epoxide, a process that allows it also to catalyze multiple glutamate residues to Gla residues in at least 17 different vitamin K–dependent proteins, thereby activating them. A second enzyme, vitamin K epoxide reductase, regenerates the vitamin K hydroquinone required for continual carboxylase activity, maintaining the cycle. Inhibition of vitamin K epoxide reductase by warfarin stops the cycle, decreasing the production of activated vitamin K–dependent proteins, including those involved in hemostasis. Vitamin K–dependent carboxylase is ubiquitous in liver and in other cells and is localized in the endoplasmic reticulum membrane, where lumenal vitamin K–dependent protein carboxylation takes place as part of the protein secretion pathway. Although vitamin K–dependent carboxylase is ubiquitous, the relative levels of different K vitamers in different tissues vary widely, and the expression of vitamin K–dependent proteins also differs. It is the common quinone ring of K vitamers that confers oxygenation activity. Vitamin K’s isoprenoid side chains of different lengths are not involved in oxygenation chemistry but may play a role in differential distribution, differential binding of the vitamers to the endoplasmic reticulum, and differential binding of vitamers to the carboxylase. Thus, all menaquinone forms and phylloquinone are competent for carboxylation, but they may have differential cooperativity with vitamin K–dependent proteins.32,100 The isoprenoid side chain of ubiquinones functions similarly in anchoring the molecule into cell membranes.13
Schurgers et al101 presented in vitro evidence suggesting that the side chain in K2 vitamins may also be regarded as a geranylgeranyl derivative that inhibits osteoclast activation, probably via inhibition of the mevalonate pathway.
Pharmacokinetics of MK-7
Shearer et al102 reviewed MK-7 absorption, distribution, metabolism, and excretion, which are all key factors in safety and efficacy. Briefly, their findings show that MK-7 is absorbed rapidly and unchanged from the small intestine via incorporation into mixed micelles comprised of bile salts, products of pancreatic lipolysis, and other dietary lipids. In the enterocytes, the mixed micelles are packaged into chylomicrons and secreted by exocytosis from the intestinal villi into the lymphatic capillaries, ultimately reaching the systemic circulation via the larger lymphatic vessels. Circulating MK-7-containing chylomicrons undergo changes in their apoprotein content that facilitate their uptake by receptor-mediated endocytosis in the liver and in bone osteoblasts, involving interactions between surface apoproteins and low-density lipoprotein receptor-related proteins. The finer details about the metabolism of MK-7 await the types of stable isotope studies that have been done for vitamin K1. However, the vitamin K epoxide cycle described above is pivotal to both the function of vitamin K and the conservation of the microsomal cellular stores of vitamin K. Humans excrete menaquinones and phylloquinone by a common degradative pathway whereby the isoprenoid side chain is first shortened to 2 major carboxylic acid metabolites with 7- and 5-carbon side chains, respectively. The metabolites are then conjugated, mainly with glucuronic acid, and excreted in the bile and urine.102
An unexpected finding in a rat feeding experiment was the presence of MK-7 epoxide in the serum of ovariectomized rats that were fed a diet containing MK-7 at 201 mg/kg for 6 weeks.103 Since MK-7 is metabolized in the liver to MK-7 epoxide, which is then glucuronidated before being excreted in bile and urine, the presence of epoxide in serum may indicate the incomplete metabolism of MK-7 in the liver. The consequences of MK-7 epoxide in serum are not clear, nor is it known whether similar accumulation could occur in humans following longer-term high-level intake, but Shearer and Newman6 reported no known adverse consequences from excess vitamin K 2,3-epoxide in humans.
In a comparison of MK-7 and vitamin K1, Schurgers et al2 (see Table 32,43,48,65,107–115 for clinical protocol and dosage details) found that maximal serum concentrations of both K1 and MK-7 were seen approximately 4 hours after intake, indicating that the doses were well absorbed. This was followed by a steep decline in serum concentrations and then a second phase at 8–96 hours in which K1 declined to baseline but MK-7 remained stable for up to 4 days or more. Using the area under the curve at 24 hours, the ratio of bioavailability of MK-7:K1 was 2.5. Using the area under the curve at 96 hours, the ratio of bioavailability of MK-7:K1 was 6. The authors concluded that MK-7 has a much longer half-life than K1 (68 hours vs 1–2 hours). Both K1 and MK-7 had linear dose–response curves at 4 hours post treatment, from 0 to 500 µg; at 24 hours, there was no effect of K1 at up to 200 µg, but MK-7 at 100 µg gave an upper limit of normal range for total serum vitamin K (1.5 nM or 1 µg/L). Menaquinone-7 accumulated during the first 2 weeks until it reached a plateau level of approximately 10 nM (6 µg/L), and K1 remained slightly above the placebo values during the entire study period. Within 3 days, both K vitamins had induced a statistically significant increase in osteocalcin carboxylation, but only with MK-7 did the ratio of circulating carboxylated osteocalcin to uncarboxylated osteocalcin continue to increase during the entire study period, suggesting that, if taken on a daily basis, MK-7 at 25 µg/d is more efficacious than vitamin K1 at 100 µg/d. However, it is important to note that the model proposed by Shearer and Newman6 and Shearer et al102 for determining the bioavailability of MK-7 will require confirmation with stable isotope studies.
Adverse event reports from clinical trials with menaquinone-7 (MK-7)
| Reference . | Clinical study design . | Endpoint(s) . | No. of subjects . | Demographic characteristics . | Dose . | Length of treatment . | Adverse events . |
|---|---|---|---|---|---|---|---|
| Knapen et al (2012)109 |
|
|
|
|
|
| 3) 2 dropped out of placebo group for weight gain; no other adverse effects reported |
| Knapen et al (2013)32 | Randomized, double-blind, placebo-controlled, parallel | Effect of MK-7 on serum uc-OC and c-OC concentrations and efficacy to decrease bone loss | n = 120 treated women, n = 124 nontreated women | Healthy postmenopausal women, 55–65 y | MK-7 at 180 µg/d | 3 y | Dropout rate of 8.6%. 12 dropouts in placebo group (hair loss, brittle nails, hot flashes, knee pain, numbness in limbs, fatigue, weight gain); 9 dropouts in MK-7 (bone pain, hot flashes, rash around eyes and ears, smelly capsules, weight gain) |
| Knapen et al (2015)110 | Randomized, double-blind, placebo-controlled, parallel | Effect of MK-7 on arterial stiffness in healthy postmenopausal women | n = 120 treated women, n = 124 nontreated women | Healthy postmenopausal women, 55–65 y | MK-7 at 180 µg/d | 3 y | No effect on fasting glucose, acute-phase markers (hs-CRP, IL-6, TNF-α) or markers of endothelial dysfunction (VCAM, E-selectin, and AGE) |
| Knapen et al (2016)111 | Randomized, partly single-blind, partly open-label bioavailability | Effect of supplemental MK-7 in yogurts or capsules on fasting plasma MK-7 concentrations | n = 43 men, 64 women | Healthy men and postmenopausal women, 45–65 y | MK-7 at 71.2 µg/d (in yogurt) or 58.3 µg (in capsule) | 42 d | In yogurt-treated groups, 7 cases of satiated feeling, heartburn, stomach ache, abdominal cramps, diarrhea, and nausea attributed to increased yogurt intake |
| Inaba et al (2015)48 | Randomized, double-blind, placebo-controlled | Dose finding and efficacy of low-dose daily MK-7 supplementation to improve osteocalcin γ-carboxylation |
|
|
|
| No adverse effects associated with study products were observed |
| Theuwissen et al (2012)108 | Randomized, double-blind, placebo-controlled exploratory pilot | Estimation of dose–response effects of MK-7 supplementation on (1) carboxylation of osteocalcin and MGP and (2) thrombin generation as an indicator of safety | n = 20 men, 22 women | Healthy men and women, 18–45 y | MK-7 at 0, 10, 20 45, 90, 180, or 360 µg/d | 12 wk | No dropouts, no adverse effects on thrombin generation observed |
| Theuwissen et al (2013)107 | Randomized, double-blind, placebo-controlled | Effect of MK-7 supplementation on serum uc-OC and dp-uc-MGP |
| Healthy children, 6–19 y, healthy adults, 20–80 y, divided into age groups of 10-y increments; selected for supplementation if circulating values of uc-OC or dp-uc-MGP were significantly higher than those of young healthy adults, 20–29 y |
|
| 1 dropout due to broken leg |
| Dalmeijer et al (2012)112 | Randomized, double-blind, placebo-controlled | Effect of MK-7 on circulating dp-uc-MGP and dp-c-MGP and on total uc-MGP, uc-OC, and c-OC | N = 60 | Healthy men and healthy postmenopausal women, 40–60 y | MK-7at 0, 180, or 360 µg/d | 12 wk | 1 dropout after enrollment but prior to treatment; no adverse events, no changes over time of prothrombin time (P = 0.92). Other CVD risk factors such as blood lipid profile or blood pressure did not differ between treatments |
| Forli et al (2010)65 | Randomized, double-blind, placebo-controlled, prospective, longitudinal | Effect of MK-7 on bone mass in the 1st year after lung or heart transplant | n = 35 lung transplant patients, n = 59 heart transplant patients | Transplant patients at risk for osteoporosis; stratified by heart vs lung transplant, men and women ≤50 y vs > 50 y, and sex | MK-7 at 0 or 180 µg/d | 12 mo | 10 heart patients did not complete 12-mo follow-up; 1 in MK-7 treatment arm died from causes not connected to study. Other adverse effects not related to treatment and not different between treatment and placebo groups |
| Emaus et al (2010)43 | Randomized, double-blind, placebo-controlled | Effect of MK-7 supplementation on rate of bone loss among healthy postmenopausal women | N = 334 | Healthy women, 50–60 y; 1–5 y after menopause | MK-7 at 0 and 360 µg/d | 12 mo | 5 participants in each group sustained a fracture; 2 in treatment group had increased nocturnal hot flushes and abdominal pain, 1 in treatment had increased palpitations that ceased at study end; in placebo group, 4 reports of muscular pain and general unwell feeling, 2 reports of itching |
| Caluwé et al (2014)113 | Randomized, single-blind, dose-finding intervention | Determination of optimum dose of MK-7 for activation of vitamin K–dependent MGP by measuring reduction of inactive dp-uc-MGP | N = 200 | Chronic hemodialysis patients in stable medical condition; men and women, ≥18 y | MK-7 at 360, 720, or 1080 µg, 3 times weekly | 8 wk | Gastrointestinal upset due to smell of MK-7 tablets in 9 subjects who withdrew |
| Brugè et al (2011)114 | Nonrandomized, nonblinded, bioavailability | Bioavailability of MK-7 in olive oil (MK-7 plasma levels) and effect on osteocalcin and its carboxylation status | n = 4 males, n = 8 females | Healthy adults, mean age 37 y (SD 3) | MK-7 at 0, 45, and 90 µg/d | 2 wk for each treatment, separated by 2-wk washout period | No dropouts or adverse effects reported |
| Schurgers et al (2007)2 |
| Comparison of absorption and efficacy (osteocalcin carboxylation) of synthetic vitamin K1 and natto-derived MK-7 |
| Healthy men and women 25–35 y; in trial 4, subjects were treated with individualized dose of acenocoumarol to reach target INR value of 2.0 within 3 wk, then maintained at stabilizing dose of acenocoumarol while treated with escalating doses of MK-7 or K1 |
|
| No adverse reactions in trials 1, 2, and 3; in the interaction trial, doses of K1 at 315 µg/d and of MK-7 at 130 µg/d caused significant decrease in INR from 2.0 to 1.5 (ie, MK-7 was much more potent) |
| Ozdemir et al (2013)115 | Nonrandomized prospective pilot study | Efficacy of MK-7 and calcitriol combination to reduce thalassemic osteopathy by improving bone mineral density and z score of lumbar spine | n = 12 girls, n = 8 boys | Pediatric thalassemic osteopathy patients, 3–18 y | MK-7 at 50 µg + calcitriol at 5 µg/d | 12 mo | No noncompliance or side effects observed |
| Reference . | Clinical study design . | Endpoint(s) . | No. of subjects . | Demographic characteristics . | Dose . | Length of treatment . | Adverse events . |
|---|---|---|---|---|---|---|---|
| Knapen et al (2012)109 |
|
|
|
|
|
| 3) 2 dropped out of placebo group for weight gain; no other adverse effects reported |
| Knapen et al (2013)32 | Randomized, double-blind, placebo-controlled, parallel | Effect of MK-7 on serum uc-OC and c-OC concentrations and efficacy to decrease bone loss | n = 120 treated women, n = 124 nontreated women | Healthy postmenopausal women, 55–65 y | MK-7 at 180 µg/d | 3 y | Dropout rate of 8.6%. 12 dropouts in placebo group (hair loss, brittle nails, hot flashes, knee pain, numbness in limbs, fatigue, weight gain); 9 dropouts in MK-7 (bone pain, hot flashes, rash around eyes and ears, smelly capsules, weight gain) |
| Knapen et al (2015)110 | Randomized, double-blind, placebo-controlled, parallel | Effect of MK-7 on arterial stiffness in healthy postmenopausal women | n = 120 treated women, n = 124 nontreated women | Healthy postmenopausal women, 55–65 y | MK-7 at 180 µg/d | 3 y | No effect on fasting glucose, acute-phase markers (hs-CRP, IL-6, TNF-α) or markers of endothelial dysfunction (VCAM, E-selectin, and AGE) |
| Knapen et al (2016)111 | Randomized, partly single-blind, partly open-label bioavailability | Effect of supplemental MK-7 in yogurts or capsules on fasting plasma MK-7 concentrations | n = 43 men, 64 women | Healthy men and postmenopausal women, 45–65 y | MK-7 at 71.2 µg/d (in yogurt) or 58.3 µg (in capsule) | 42 d | In yogurt-treated groups, 7 cases of satiated feeling, heartburn, stomach ache, abdominal cramps, diarrhea, and nausea attributed to increased yogurt intake |
| Inaba et al (2015)48 | Randomized, double-blind, placebo-controlled | Dose finding and efficacy of low-dose daily MK-7 supplementation to improve osteocalcin γ-carboxylation |
|
|
|
| No adverse effects associated with study products were observed |
| Theuwissen et al (2012)108 | Randomized, double-blind, placebo-controlled exploratory pilot | Estimation of dose–response effects of MK-7 supplementation on (1) carboxylation of osteocalcin and MGP and (2) thrombin generation as an indicator of safety | n = 20 men, 22 women | Healthy men and women, 18–45 y | MK-7 at 0, 10, 20 45, 90, 180, or 360 µg/d | 12 wk | No dropouts, no adverse effects on thrombin generation observed |
| Theuwissen et al (2013)107 | Randomized, double-blind, placebo-controlled | Effect of MK-7 supplementation on serum uc-OC and dp-uc-MGP |
| Healthy children, 6–19 y, healthy adults, 20–80 y, divided into age groups of 10-y increments; selected for supplementation if circulating values of uc-OC or dp-uc-MGP were significantly higher than those of young healthy adults, 20–29 y |
|
| 1 dropout due to broken leg |
| Dalmeijer et al (2012)112 | Randomized, double-blind, placebo-controlled | Effect of MK-7 on circulating dp-uc-MGP and dp-c-MGP and on total uc-MGP, uc-OC, and c-OC | N = 60 | Healthy men and healthy postmenopausal women, 40–60 y | MK-7at 0, 180, or 360 µg/d | 12 wk | 1 dropout after enrollment but prior to treatment; no adverse events, no changes over time of prothrombin time (P = 0.92). Other CVD risk factors such as blood lipid profile or blood pressure did not differ between treatments |
| Forli et al (2010)65 | Randomized, double-blind, placebo-controlled, prospective, longitudinal | Effect of MK-7 on bone mass in the 1st year after lung or heart transplant | n = 35 lung transplant patients, n = 59 heart transplant patients | Transplant patients at risk for osteoporosis; stratified by heart vs lung transplant, men and women ≤50 y vs > 50 y, and sex | MK-7 at 0 or 180 µg/d | 12 mo | 10 heart patients did not complete 12-mo follow-up; 1 in MK-7 treatment arm died from causes not connected to study. Other adverse effects not related to treatment and not different between treatment and placebo groups |
| Emaus et al (2010)43 | Randomized, double-blind, placebo-controlled | Effect of MK-7 supplementation on rate of bone loss among healthy postmenopausal women | N = 334 | Healthy women, 50–60 y; 1–5 y after menopause | MK-7 at 0 and 360 µg/d | 12 mo | 5 participants in each group sustained a fracture; 2 in treatment group had increased nocturnal hot flushes and abdominal pain, 1 in treatment had increased palpitations that ceased at study end; in placebo group, 4 reports of muscular pain and general unwell feeling, 2 reports of itching |
| Caluwé et al (2014)113 | Randomized, single-blind, dose-finding intervention | Determination of optimum dose of MK-7 for activation of vitamin K–dependent MGP by measuring reduction of inactive dp-uc-MGP | N = 200 | Chronic hemodialysis patients in stable medical condition; men and women, ≥18 y | MK-7 at 360, 720, or 1080 µg, 3 times weekly | 8 wk | Gastrointestinal upset due to smell of MK-7 tablets in 9 subjects who withdrew |
| Brugè et al (2011)114 | Nonrandomized, nonblinded, bioavailability | Bioavailability of MK-7 in olive oil (MK-7 plasma levels) and effect on osteocalcin and its carboxylation status | n = 4 males, n = 8 females | Healthy adults, mean age 37 y (SD 3) | MK-7 at 0, 45, and 90 µg/d | 2 wk for each treatment, separated by 2-wk washout period | No dropouts or adverse effects reported |
| Schurgers et al (2007)2 |
| Comparison of absorption and efficacy (osteocalcin carboxylation) of synthetic vitamin K1 and natto-derived MK-7 |
| Healthy men and women 25–35 y; in trial 4, subjects were treated with individualized dose of acenocoumarol to reach target INR value of 2.0 within 3 wk, then maintained at stabilizing dose of acenocoumarol while treated with escalating doses of MK-7 or K1 |
|
| No adverse reactions in trials 1, 2, and 3; in the interaction trial, doses of K1 at 315 µg/d and of MK-7 at 130 µg/d caused significant decrease in INR from 2.0 to 1.5 (ie, MK-7 was much more potent) |
| Ozdemir et al (2013)115 | Nonrandomized prospective pilot study | Efficacy of MK-7 and calcitriol combination to reduce thalassemic osteopathy by improving bone mineral density and z score of lumbar spine | n = 12 girls, n = 8 boys | Pediatric thalassemic osteopathy patients, 3–18 y | MK-7 at 50 µg + calcitriol at 5 µg/d | 12 mo | No noncompliance or side effects observed |
Abbreviations: AGE, advanced glycation endproducts; c-OC, carboxylated osteocalcin; CVD, cardiovascular disease; dc-uc-MGP, desphospho-uncarboxylated matrix Gla protein; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin 6, INR, international normalized ratio; MGP, matrix Gla protein; SD, standard deviation; TNF-α, tumor necrosis factor α; uc-OC, uncarboxylated osteocalcin; VCAM, vascular cell adhesion molecule.
Adverse event reports from clinical trials with menaquinone-7 (MK-7)
| Reference . | Clinical study design . | Endpoint(s) . | No. of subjects . | Demographic characteristics . | Dose . | Length of treatment . | Adverse events . |
|---|---|---|---|---|---|---|---|
| Knapen et al (2012)109 |
|
|
|
|
|
| 3) 2 dropped out of placebo group for weight gain; no other adverse effects reported |
| Knapen et al (2013)32 | Randomized, double-blind, placebo-controlled, parallel | Effect of MK-7 on serum uc-OC and c-OC concentrations and efficacy to decrease bone loss | n = 120 treated women, n = 124 nontreated women | Healthy postmenopausal women, 55–65 y | MK-7 at 180 µg/d | 3 y | Dropout rate of 8.6%. 12 dropouts in placebo group (hair loss, brittle nails, hot flashes, knee pain, numbness in limbs, fatigue, weight gain); 9 dropouts in MK-7 (bone pain, hot flashes, rash around eyes and ears, smelly capsules, weight gain) |
| Knapen et al (2015)110 | Randomized, double-blind, placebo-controlled, parallel | Effect of MK-7 on arterial stiffness in healthy postmenopausal women | n = 120 treated women, n = 124 nontreated women | Healthy postmenopausal women, 55–65 y | MK-7 at 180 µg/d | 3 y | No effect on fasting glucose, acute-phase markers (hs-CRP, IL-6, TNF-α) or markers of endothelial dysfunction (VCAM, E-selectin, and AGE) |
| Knapen et al (2016)111 | Randomized, partly single-blind, partly open-label bioavailability | Effect of supplemental MK-7 in yogurts or capsules on fasting plasma MK-7 concentrations | n = 43 men, 64 women | Healthy men and postmenopausal women, 45–65 y | MK-7 at 71.2 µg/d (in yogurt) or 58.3 µg (in capsule) | 42 d | In yogurt-treated groups, 7 cases of satiated feeling, heartburn, stomach ache, abdominal cramps, diarrhea, and nausea attributed to increased yogurt intake |
| Inaba et al (2015)48 | Randomized, double-blind, placebo-controlled | Dose finding and efficacy of low-dose daily MK-7 supplementation to improve osteocalcin γ-carboxylation |
|
|
|
| No adverse effects associated with study products were observed |
| Theuwissen et al (2012)108 | Randomized, double-blind, placebo-controlled exploratory pilot | Estimation of dose–response effects of MK-7 supplementation on (1) carboxylation of osteocalcin and MGP and (2) thrombin generation as an indicator of safety | n = 20 men, 22 women | Healthy men and women, 18–45 y | MK-7 at 0, 10, 20 45, 90, 180, or 360 µg/d | 12 wk | No dropouts, no adverse effects on thrombin generation observed |
| Theuwissen et al (2013)107 | Randomized, double-blind, placebo-controlled | Effect of MK-7 supplementation on serum uc-OC and dp-uc-MGP |
| Healthy children, 6–19 y, healthy adults, 20–80 y, divided into age groups of 10-y increments; selected for supplementation if circulating values of uc-OC or dp-uc-MGP were significantly higher than those of young healthy adults, 20–29 y |
|
| 1 dropout due to broken leg |
| Dalmeijer et al (2012)112 | Randomized, double-blind, placebo-controlled | Effect of MK-7 on circulating dp-uc-MGP and dp-c-MGP and on total uc-MGP, uc-OC, and c-OC | N = 60 | Healthy men and healthy postmenopausal women, 40–60 y | MK-7at 0, 180, or 360 µg/d | 12 wk | 1 dropout after enrollment but prior to treatment; no adverse events, no changes over time of prothrombin time (P = 0.92). Other CVD risk factors such as blood lipid profile or blood pressure did not differ between treatments |
| Forli et al (2010)65 | Randomized, double-blind, placebo-controlled, prospective, longitudinal | Effect of MK-7 on bone mass in the 1st year after lung or heart transplant | n = 35 lung transplant patients, n = 59 heart transplant patients | Transplant patients at risk for osteoporosis; stratified by heart vs lung transplant, men and women ≤50 y vs > 50 y, and sex | MK-7 at 0 or 180 µg/d | 12 mo | 10 heart patients did not complete 12-mo follow-up; 1 in MK-7 treatment arm died from causes not connected to study. Other adverse effects not related to treatment and not different between treatment and placebo groups |
| Emaus et al (2010)43 | Randomized, double-blind, placebo-controlled | Effect of MK-7 supplementation on rate of bone loss among healthy postmenopausal women | N = 334 | Healthy women, 50–60 y; 1–5 y after menopause | MK-7 at 0 and 360 µg/d | 12 mo | 5 participants in each group sustained a fracture; 2 in treatment group had increased nocturnal hot flushes and abdominal pain, 1 in treatment had increased palpitations that ceased at study end; in placebo group, 4 reports of muscular pain and general unwell feeling, 2 reports of itching |
| Caluwé et al (2014)113 | Randomized, single-blind, dose-finding intervention | Determination of optimum dose of MK-7 for activation of vitamin K–dependent MGP by measuring reduction of inactive dp-uc-MGP | N = 200 | Chronic hemodialysis patients in stable medical condition; men and women, ≥18 y | MK-7 at 360, 720, or 1080 µg, 3 times weekly | 8 wk | Gastrointestinal upset due to smell of MK-7 tablets in 9 subjects who withdrew |
| Brugè et al (2011)114 | Nonrandomized, nonblinded, bioavailability | Bioavailability of MK-7 in olive oil (MK-7 plasma levels) and effect on osteocalcin and its carboxylation status | n = 4 males, n = 8 females | Healthy adults, mean age 37 y (SD 3) | MK-7 at 0, 45, and 90 µg/d | 2 wk for each treatment, separated by 2-wk washout period | No dropouts or adverse effects reported |
| Schurgers et al (2007)2 |
| Comparison of absorption and efficacy (osteocalcin carboxylation) of synthetic vitamin K1 and natto-derived MK-7 |
| Healthy men and women 25–35 y; in trial 4, subjects were treated with individualized dose of acenocoumarol to reach target INR value of 2.0 within 3 wk, then maintained at stabilizing dose of acenocoumarol while treated with escalating doses of MK-7 or K1 |
|
| No adverse reactions in trials 1, 2, and 3; in the interaction trial, doses of K1 at 315 µg/d and of MK-7 at 130 µg/d caused significant decrease in INR from 2.0 to 1.5 (ie, MK-7 was much more potent) |
| Ozdemir et al (2013)115 | Nonrandomized prospective pilot study | Efficacy of MK-7 and calcitriol combination to reduce thalassemic osteopathy by improving bone mineral density and z score of lumbar spine | n = 12 girls, n = 8 boys | Pediatric thalassemic osteopathy patients, 3–18 y | MK-7 at 50 µg + calcitriol at 5 µg/d | 12 mo | No noncompliance or side effects observed |
| Reference . | Clinical study design . | Endpoint(s) . | No. of subjects . | Demographic characteristics . | Dose . | Length of treatment . | Adverse events . |
|---|---|---|---|---|---|---|---|
| Knapen et al (2012)109 |
|
|
|
|
|
| 3) 2 dropped out of placebo group for weight gain; no other adverse effects reported |
| Knapen et al (2013)32 | Randomized, double-blind, placebo-controlled, parallel | Effect of MK-7 on serum uc-OC and c-OC concentrations and efficacy to decrease bone loss | n = 120 treated women, n = 124 nontreated women | Healthy postmenopausal women, 55–65 y | MK-7 at 180 µg/d | 3 y | Dropout rate of 8.6%. 12 dropouts in placebo group (hair loss, brittle nails, hot flashes, knee pain, numbness in limbs, fatigue, weight gain); 9 dropouts in MK-7 (bone pain, hot flashes, rash around eyes and ears, smelly capsules, weight gain) |
| Knapen et al (2015)110 | Randomized, double-blind, placebo-controlled, parallel | Effect of MK-7 on arterial stiffness in healthy postmenopausal women | n = 120 treated women, n = 124 nontreated women | Healthy postmenopausal women, 55–65 y | MK-7 at 180 µg/d | 3 y | No effect on fasting glucose, acute-phase markers (hs-CRP, IL-6, TNF-α) or markers of endothelial dysfunction (VCAM, E-selectin, and AGE) |
| Knapen et al (2016)111 | Randomized, partly single-blind, partly open-label bioavailability | Effect of supplemental MK-7 in yogurts or capsules on fasting plasma MK-7 concentrations | n = 43 men, 64 women | Healthy men and postmenopausal women, 45–65 y | MK-7 at 71.2 µg/d (in yogurt) or 58.3 µg (in capsule) | 42 d | In yogurt-treated groups, 7 cases of satiated feeling, heartburn, stomach ache, abdominal cramps, diarrhea, and nausea attributed to increased yogurt intake |
| Inaba et al (2015)48 | Randomized, double-blind, placebo-controlled | Dose finding and efficacy of low-dose daily MK-7 supplementation to improve osteocalcin γ-carboxylation |
|
|
|
| No adverse effects associated with study products were observed |
| Theuwissen et al (2012)108 | Randomized, double-blind, placebo-controlled exploratory pilot | Estimation of dose–response effects of MK-7 supplementation on (1) carboxylation of osteocalcin and MGP and (2) thrombin generation as an indicator of safety | n = 20 men, 22 women | Healthy men and women, 18–45 y | MK-7 at 0, 10, 20 45, 90, 180, or 360 µg/d | 12 wk | No dropouts, no adverse effects on thrombin generation observed |
| Theuwissen et al (2013)107 | Randomized, double-blind, placebo-controlled | Effect of MK-7 supplementation on serum uc-OC and dp-uc-MGP |
| Healthy children, 6–19 y, healthy adults, 20–80 y, divided into age groups of 10-y increments; selected for supplementation if circulating values of uc-OC or dp-uc-MGP were significantly higher than those of young healthy adults, 20–29 y |
|
| 1 dropout due to broken leg |
| Dalmeijer et al (2012)112 | Randomized, double-blind, placebo-controlled | Effect of MK-7 on circulating dp-uc-MGP and dp-c-MGP and on total uc-MGP, uc-OC, and c-OC | N = 60 | Healthy men and healthy postmenopausal women, 40–60 y | MK-7at 0, 180, or 360 µg/d | 12 wk | 1 dropout after enrollment but prior to treatment; no adverse events, no changes over time of prothrombin time (P = 0.92). Other CVD risk factors such as blood lipid profile or blood pressure did not differ between treatments |
| Forli et al (2010)65 | Randomized, double-blind, placebo-controlled, prospective, longitudinal | Effect of MK-7 on bone mass in the 1st year after lung or heart transplant | n = 35 lung transplant patients, n = 59 heart transplant patients | Transplant patients at risk for osteoporosis; stratified by heart vs lung transplant, men and women ≤50 y vs > 50 y, and sex | MK-7 at 0 or 180 µg/d | 12 mo | 10 heart patients did not complete 12-mo follow-up; 1 in MK-7 treatment arm died from causes not connected to study. Other adverse effects not related to treatment and not different between treatment and placebo groups |
| Emaus et al (2010)43 | Randomized, double-blind, placebo-controlled | Effect of MK-7 supplementation on rate of bone loss among healthy postmenopausal women | N = 334 | Healthy women, 50–60 y; 1–5 y after menopause | MK-7 at 0 and 360 µg/d | 12 mo | 5 participants in each group sustained a fracture; 2 in treatment group had increased nocturnal hot flushes and abdominal pain, 1 in treatment had increased palpitations that ceased at study end; in placebo group, 4 reports of muscular pain and general unwell feeling, 2 reports of itching |
| Caluwé et al (2014)113 | Randomized, single-blind, dose-finding intervention | Determination of optimum dose of MK-7 for activation of vitamin K–dependent MGP by measuring reduction of inactive dp-uc-MGP | N = 200 | Chronic hemodialysis patients in stable medical condition; men and women, ≥18 y | MK-7 at 360, 720, or 1080 µg, 3 times weekly | 8 wk | Gastrointestinal upset due to smell of MK-7 tablets in 9 subjects who withdrew |
| Brugè et al (2011)114 | Nonrandomized, nonblinded, bioavailability | Bioavailability of MK-7 in olive oil (MK-7 plasma levels) and effect on osteocalcin and its carboxylation status | n = 4 males, n = 8 females | Healthy adults, mean age 37 y (SD 3) | MK-7 at 0, 45, and 90 µg/d | 2 wk for each treatment, separated by 2-wk washout period | No dropouts or adverse effects reported |
| Schurgers et al (2007)2 |
| Comparison of absorption and efficacy (osteocalcin carboxylation) of synthetic vitamin K1 and natto-derived MK-7 |
| Healthy men and women 25–35 y; in trial 4, subjects were treated with individualized dose of acenocoumarol to reach target INR value of 2.0 within 3 wk, then maintained at stabilizing dose of acenocoumarol while treated with escalating doses of MK-7 or K1 |
|
| No adverse reactions in trials 1, 2, and 3; in the interaction trial, doses of K1 at 315 µg/d and of MK-7 at 130 µg/d caused significant decrease in INR from 2.0 to 1.5 (ie, MK-7 was much more potent) |
| Ozdemir et al (2013)115 | Nonrandomized prospective pilot study | Efficacy of MK-7 and calcitriol combination to reduce thalassemic osteopathy by improving bone mineral density and z score of lumbar spine | n = 12 girls, n = 8 boys | Pediatric thalassemic osteopathy patients, 3–18 y | MK-7 at 50 µg + calcitriol at 5 µg/d | 12 mo | No noncompliance or side effects observed |
Abbreviations: AGE, advanced glycation endproducts; c-OC, carboxylated osteocalcin; CVD, cardiovascular disease; dc-uc-MGP, desphospho-uncarboxylated matrix Gla protein; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin 6, INR, international normalized ratio; MGP, matrix Gla protein; SD, standard deviation; TNF-α, tumor necrosis factor α; uc-OC, uncarboxylated osteocalcin; VCAM, vascular cell adhesion molecule.
Schurgers et al2 also reported that, on a molar basis, MK-7 is 3 to 4 times more potent at interfering with the action of oral anticoagulant drugs, and by weight the effect of MK-7 is approximately 2.5 times that of vitamin K1. The conclusion that MK-7 doses of more than 50 µg/d may interfere with anticoagulant treatment in a clinically relevant way is therefore based on these results of the potency of MK-7 compared with that of vitamin K1 and extrapolation of findings from a previous study in which supplements containing no more than 100 µg/d of vitamin K1 were not likely to result in clinically relevant disturbances of oral anticoagulant therapy in healthy individuals. The studies conducted by Schurgers et al2 were not designed or powered to assess safety.
In summary, bioavailability among the various forms of menaquinones appears to be related in part to the length of the side chain, as menaquinones with long side chains (eg, MK-7, MK-8, and MK-9) are better absorbed from food compared with MK-4, which has a short side chain.2,79,104
Adverse effect reports from clinical trials of MK-7
A search of the US National Institutes of Health clinical trials database yielded 34 trials using the search words “menaquinone-7” or “MK-7,” including at least 7 trials not yet completed.105 A similar search of the WHO International Clinical Trials Registry Platform resulted in a list of 18 trials, 6 of which are not in the National Institutes of Health database.106 These results, from only 2 of the international clinical trial registries, are an indication of the active, ongoing human clinical research on MK-7.
Table 3 summarizes reports of adverse effects from peer-reviewed publications2,43,48,65,107–115 of human clinical trials of MK-7 not already evaluated by the EFSA Panel.24 Published clinical trials that made no mention of whether adverse events occurred or of any other aspects of safety are excluded because an evaluation of efficacy was not within the objective of this review. Reports of clinical trials on other vitamers of K2 or of K1 are also excluded.
Table 3 shows that treatment with MK-7 at levels of up to 180 µg/d for 3 years, of up to 360 µg/d for 12 weeks, or of up to 1080 µg thrice weekly for 8 weeks in treatment populations of up to 120 individuals was associated with no significant adverse effects compared with placebo. Adverse effects specifically attributed to MK-7 were limited to gastrointestinal upset associated with the product’s smell.
Cockayne et al,38 in a systematic review and meta-analysis of 13 randomized controlled trials of vitamins K1 and K2 supplementation for the prevention of bone fractures, found no study reporting any serious adverse events associated with vitamin K. Minor gastrointestinal problems were reported by some authors, but this type of clinical trial adverse event is almost ubiquitous.
Pharmacovigilance: adverse event reports for MK-7
Adverse event report information was sought from published case reports or reviews, the FDA MedWatch program,116 the Canada Vigilance Adverse Reaction Online Database,117 the UK Medicines and Healthcare products Regulatory Agency Drug Analysis Prints A–Z,118 the Australian Therapeutic Goods Administration Database of Adverse Event Notifications—medicines,119 and the New Zealand MedSafe Suspected Medicine Adverse Reaction Search.120
Because the MedWatch program focuses on adverse event reports for specific products rather than for ingredients, no reports of adverse reactions associated with MK-7 could be retrieved.116 The Canada Vigilance Program database117 had no entries for products named menaquinone-7 or MK-7, but the search term “menaquinones” retrieved 1 adverse event report. The report concerned a nonserious event of diarrhea and vomiting associated with a “suspected” 92-ingredient product containing only 6 µg of vitamin K2 (vitamer not specified) per daily dose.88 The UK Medicines and Healthcare products Regulatory Agency’s Drug Analysis Prints database contained no adverse event reports for MK-7 or products containing MK-7, only for menadiol sodium diphosphate and phytonadione.118 The Australian Therapeutic Goods Administration Database of Adverse Event Notifications—medicines119 had 2 adverse event reports associated with suspected products containing menaquinones. One case of “hot flush” and blurred vision was associated with a Listed Medicine product called K2-180, which is a single-ingredient capsule containing 180 µg of MK-7 per capsule. The other was a case of myocardial infarction and splenic infarction associated with an unspecified menaquinone-containing product taken in combination with multiple prescription drugs that included human prothrombin complex (also a suspected product in the adverse event) and with nonprescription drugs that included acetylsalicylic acid. The New Zealand MedSafe Suspected Medicine Adverse Reaction Search did not contain any adverse event reports associated with menaquinones.120
Drug interactions between MK-7 and anticoagulants
Evidence suggests that alterations in the intake of vitamin K can affect the response to anticoagulant agents. Theuwissen et al107 anticoagulated 18 healthy men and women with acenocoumarol, and 15 of them attained a target international normalized ratio (INR) of 2.0. Over 6 subsequent weeks, participants were given increasing doses of MK-7 (10, 20, and 4 µg/d) while continuing individualized acenocoumarol treatment. Besides increasing the INR, acenocoumarol treatment significantly increased the levels of uncarboxylated factor II, uncarboxylated osteocalcin, and desphospho-uncarboxylated matrix Gla protein and decreased endogenous thrombin generation. A daily intake of 45 µg of MK-7 significantly decreased the group mean values of both the INR and uncarboxylated factor II by approximately 40%. Daily intakes of 10 and 20 µg of MK-7 were independently judged by 2 hematologists to cause a clinically relevant lowering of the INR in at least 40% and 60% of participants, respectively, and to significantly increase endogenous thrombin generation by approximately 20% and approximately 30%, respectively. Circulating uncarboxylated osteocalcin and desphospho-uncarboxylated matrix Gla protein were not affected by MK-7 intake. In conclusion, MK-7 supplementation at doses as low as 10 µg (lower than the usual retail dose of 45 µg) significantly influenced anticoagulation sensitivity in some individuals.
In contrast, healthy patients (N = 42 men and women aged 18–45 years) treated with doses of 0, 10, 20, 45, 90, 180, or 360 µg of MK-7 once daily with a meal for 12 weeks showed improved carboxylation of extrahepatic vitamin K–dependent proteins and no adverse effects on thrombin generation.108 A comparison of findings by Theuwissen et al108 and Theuwissen et al107 indicates that the risk for hemostatic system–related adverse effects is primarily associated with vulnerable populations taking coumarin-type anticoagulant drugs.
Interestingly, Sconce et al121 noted that patients with unstable control of anticoagulation (N =26, with an INR value standard deviation of >0.5 and with ≥3 warfarin dose changes in the previous 6 months) had significantly (P < 0.001) lower dietary intakes of vitamin K (primarily K1, based on information from food composition databases) than those with stable control (N = 26). They suggested that daily supplementation with oral vitamin K in unstable patients could lead to a more stable anticoagulation response to warfarin. The same research group then conducted a double-blind trial (N = 70) in warfarin-treated patients with unstable anticoagulant control, randomizing them to receive 150 µg of oral vitamin K1 or placebo for 6 months.122 Phylloquinone supplementation resulted in a significantly (P < 0.001) greater decrease in the standard deviation of INR and a significantly (P < 0.01) greater increase in the percentage time within the target INR range. Anticoagulation control improved in 33 of 35 patients receiving vitamin K1; 19 patients achieved good anticoagulant control. However, only 24 of 33 patients receiving placebo showed some degree of improvement, and only 7 of 33 achieved good anticoagulant control.
Li et al,123 who determined dietary vitamin K intake and anticoagulation control during the initiation phase of warfarin therapy as part of a prospective cohort study (N = 368), also concluded that moderate vitamin K intake may be optimal when initiating warfarin. Gebuis et al124 conducted a randomized, double-blind, placebo-controlled dose-finding study (N = 400) with vitamin K1 to improve the stability of anticoagulation therapy and reported that patients from both the 100 µg/d group and the 150 µg/d group had a 2-fold higher chance of reaching an INR within the therapeutic range at least 85% of time. There were no differences in thromboembolic or hemorrhagic complications between the groups. Similarly, Majeed et al125 found that supplementation of patients having unstable anticoagulation control (N =26 treatment, 24 placebo) with vitamin K1 at 200 µg/d significantly (P = 0.026) reduced the standard deviation of INRs. However, Zuchinali et al126 reported genetic polymorphisms of the vitamin K epoxide reductase complex subunit 1 gene in some patients, which may partly explain significant interindividual variability of the effect of vitamin K to reverse overanticoagulation.
In summary, there is a risk of interaction between MK-7 or other forms of vitamin K and warfarin or other anticoagulant therapy. However, through dose titration and patient counseling, the physician or pharmacist may be able to mitigate that risk to maintain stable anticoagulation control, so long as the patient’s vitamin K intake is known.
Products in the Dietary Supplement Label Database and the Licensed Natural Health Products Database that contain menaquinones are all labeled as vitamin K2. Only by examining the product labels is it possible to identify those that contain MK-7. An important step in mitigating risk will be to have all products containing MK-7 continue to be labeled as a form of vitamin K.
Safety of MK-7 with regard to food allergies
Menaquinone-7 products are made by fermentation of soy protein. The US Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) identifies soybeans as a “major food allergen.”127 The Canadian Food and Drug Regulations128 have very similar provisions for the labeling of soy as a food allergen. The FALCPA does not require the FDA to establish a threshold level for any food allergen, but it excludes from the labeling provisions any highly refined oil derived from a major food allergen. Health Canada’s position is similar (ie, the regulations for enhanced labeling of food allergens do not apply to highly refined oils).
The FDA document Approaches to Establish Thresholds for Major Food Allergens and for Gluten in Food129 and the Health Canada document Health Canada’s Position on Highly Refined Oils Derived from Food Allergen Sources130 both note that the consumption of highly refined oils derived from major food allergens by allergic individuals does not appear to be associated with allergic reactions. The mean protein content of highly refined soybean oil is 0.74 µg/mL, with a standard deviation of 1.3 µg/mL. The range of published LOAELs for soy as a food allergen is 88–522 mg of protein.129
Even if MK-7 is purified to more than 99%, such that any carryover soy protein is at a concentration below a limit of quantitation of 2.5 mg/kg (2.5 ppm) and is thus within the range found not to be problematic for allergic consumers, labeling of MK-7 preparations as a form of vitamin K2 derived from soy protein is advisable to allow consumers to make informed choices about protecting their health. Menaquinone-7 finished products are not highly refined vegetable oil products and thus will not be covered by the exemptions in the US and Canadian regulations. The FALCPA states that any person can petition the Secretary of Health and Human Services for an exemption. The notification process must include scientific evidence (including the analytical method used) demonstrating that the food ingredient (as derived by the production method specified in the notification) does not contain allergenic protein (eg, in this case, by showing that the purification of MK-7 is sufficient to eliminate the presence of soy protein).
Nevertheless, some consumers with food allergies are sensitive to minute amounts of the allergenic protein, and carryover of substrate proteins from fermentations to finished products that should be free of those proteins is an established risk factor for allergic reactions (eg, carryover of soy and milk proteins from the fermentation of live microorganisms made into probiotic products that are labeled as being free of these major food allergens131). Therefore, it is in the best interest of consumers and of industry that MK-7 products be labeled as manufactured with soy protein, even if the finished product has been highly purified.
Supportive in vitro and animal toxicological studies
In support of the human safety data, Table 430,132 and Table 51,32 summarize studies that have not been described in reviews already cited in this article, such as the EFSA safety evaluation.24,Table 4 summarizes published animal acute and subchronic toxicity studies with MK-7, and Table 5 summarizes genotoxicity, in vitro mutagenicity, and carcinogenicity studies with MK-7. The general conclusions that can be drawn from the data in these tables are that MK-7 possesses a low acute oral toxicity, did not cause significant adverse effects in 90-day subchronic oral toxicity studies at doses several times higher than average estimated intake levels, and was not genotoxic or mutagenic.30,132
Animal acute and subchronic toxicity studies with menaquinone-7 (MK-7)
| Reference . | Study design . | Observations . | Results . |
|---|---|---|---|
| Ravishankar et al (2015)132 | Acute oral toxicity study in rats. MK-7 suspended in a propylene glycol vehicle administered orally by gavage at 0.5 mg/kg, 1.0 mg/kg, 10 mg/kg, or 20 mg/kg. MK-7 or placebo containing propylene glycol only administered once daily for 14 d | Rats were monitored for general behavior, toxic signs and symptoms, or mortality during the experimental period. At end of study, mice were killed and examined for gross necropsy performed in vital organs | No effect of MK-7 on food and water consumption, no physical or behavioral changes, and no mortality observed in any group after 14 d. In the 1-mg/kg group, 2 of 8 animals had mild irritability. No statistically significant difference in body weight gain observed in any group. No adverse effects observed in either sex in any group. All rats survived, with no symptoms of distress or toxic effects. LD50 was > 2000 mg/kg body weight |
| Ravishankar et al (2015)132 | Subchronic oral toxicity study in rats. MK-7 administered orally by gavage at 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg. MK-7 or placebo containing propylene glycol only given once daily for 90 d. MK-7 prepared fresh daily and administered as 1 mL/100 g body weight between 8 am and 9 am | Rats were monitored for changes in behavior, mortality, body weight, and food consumption. Blood was collected on days 15, 45, and 91 (at sacrifice) to determine fasting blood sugar; levels of serum urea, creatinine, uric acid, total cholesterol, triglycerides, total protein, and serum calcium; albumin-to-globulin ratio, liver enzymes SGOT and SGPT, and alkaline phosphatase activity. Other hematologic parameters measured on those days included total WBC count, total lymphocyte count, total monocyte count, total granulocyte count, lymphocyte percentage, monocyte percentage, granulocyte percentage, RBC count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, RBC distribution width, and clotting time. Urine specific gravity and pH were measured at days 15, 45, and 91 (at sacrifice). After 90 d, rats were killed and autopsied, and histological studies of brain, pituitary, thymus, lymph node, heart, lungs, spleen, seminal vesicles, uterus, skin, trachea, liver, stomach, jejunum, kidney, testis, prostate, and ovary were performed | Normal weight gain pattern in all groups; slight increased weight gain in rats receiving MK-7, but increase was not statistically significant. Male and female rats showed significant weight increase at 90 d in all treatment group compared with controls. Average weights of organs in male and female rats were not significantly different from those in controls (liver, thymus, kidney, spleen, testis, seminal vesicles, prostate, and uterus). However, female rats in the 0.5-mg/kg group had a statistically significant decrease in heart weight compared with controls. Liver enzymes (SGPT, SGOT, alkaline phosphatase) and, similarly, serum glucose, total protein, creatinine, and blood urea levels showed no significant changes in any group. Uric acid levels were not changed in females, but in males there was a significant decrease at day 45 in the 0.1-mg/kg group. Conversely, levels in both sexes were increased significantly at day 45 and day 90 in the 0.5-mg/kg and 1.0-mg/kg groups. Blood counts (total WBC count, RBC count, and hematocrit) were significantly decreased at day 90 in both sexes; hemoglobin levels were generally the same except in males on day 45 in the 0.1-mg/kg group (increased), on day 45 in the 1.0-mg/kg group (decreased), and in control females (decreased). Mean corpuscular hemoglobin concentration, corpuscular volume, and RBC distribution width values and clotting time were not affected. Urinalysis showed no significant changes in specific gravity or pH. Histopathological study showed no remarkable changes in organs of control or treated animals except in females, in which proliferation of uterine epithelium was seen at all levels in 1–2 rats, while cytoarchitecture was normal in all other rats. Significant levels at P < 0.05 |
| Pucaj et al (2011)30 | Acute oral toxicity test. MK-7 suspended in sunflower oil was administered to mice by single oral gavage to achieve a dose of 2000 mg/kg body weight | Mice were weighed at days 0, 7, and 14 (termination). Animals were monitored twice daily on the day of dosing and once daily thereafter. Observations included changes in skin, fur, eyes, mucous membranes, and respiratory, circulatory, autonomic, and central nervous systems. Animals were also observed for changes in motor activity and behavior pattern | At limit dose level of 2000 mg/kg, MK-7 did not induce any signs of toxicity in any of the treated mice following dosing or during the 14-d observation period. Body weight gain of treated mice was not adversely affected. Median LD50 was > 2000 mg/kg body weight |
| Pucaj et al (2011)30 | Subchronic oral study. Rats were given MK-7 for 90 d at doses of 0, 2.5, 5.0, and 10 mg/kg body weight per day | Rats were observed for clinical signs and mortality twice daily throughout the study and for reaction to treatment such as changes in skin, fur, eyes, and mucous membranes. Rats were also monitored for changes in respiratory, circulatory, autonomic, and central nervous systems, for changes in somatomotor activity and behavior patterns, and for any other signs of ill health. Terminal body weights were recorded on day 91–92 for main study animals. During the recovery, animal weights were determined. Hematologic and clinical chemistry data of rats were obtained and compared with baseline data | No deaths occurred, and no compound-related toxicity was indicated by clinical observations or by ophthalmology, clinical pathology, gross necropsy, or histopathology. Any statistical significant differences in clinical pathology parameters and/or organ weights noted were considered to be within normal biological variability. Median LD50 = 2000 mg/kg and NOAEL = 10 mg/kg body weight per day |
| Reference . | Study design . | Observations . | Results . |
|---|---|---|---|
| Ravishankar et al (2015)132 | Acute oral toxicity study in rats. MK-7 suspended in a propylene glycol vehicle administered orally by gavage at 0.5 mg/kg, 1.0 mg/kg, 10 mg/kg, or 20 mg/kg. MK-7 or placebo containing propylene glycol only administered once daily for 14 d | Rats were monitored for general behavior, toxic signs and symptoms, or mortality during the experimental period. At end of study, mice were killed and examined for gross necropsy performed in vital organs | No effect of MK-7 on food and water consumption, no physical or behavioral changes, and no mortality observed in any group after 14 d. In the 1-mg/kg group, 2 of 8 animals had mild irritability. No statistically significant difference in body weight gain observed in any group. No adverse effects observed in either sex in any group. All rats survived, with no symptoms of distress or toxic effects. LD50 was > 2000 mg/kg body weight |
| Ravishankar et al (2015)132 | Subchronic oral toxicity study in rats. MK-7 administered orally by gavage at 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg. MK-7 or placebo containing propylene glycol only given once daily for 90 d. MK-7 prepared fresh daily and administered as 1 mL/100 g body weight between 8 am and 9 am | Rats were monitored for changes in behavior, mortality, body weight, and food consumption. Blood was collected on days 15, 45, and 91 (at sacrifice) to determine fasting blood sugar; levels of serum urea, creatinine, uric acid, total cholesterol, triglycerides, total protein, and serum calcium; albumin-to-globulin ratio, liver enzymes SGOT and SGPT, and alkaline phosphatase activity. Other hematologic parameters measured on those days included total WBC count, total lymphocyte count, total monocyte count, total granulocyte count, lymphocyte percentage, monocyte percentage, granulocyte percentage, RBC count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, RBC distribution width, and clotting time. Urine specific gravity and pH were measured at days 15, 45, and 91 (at sacrifice). After 90 d, rats were killed and autopsied, and histological studies of brain, pituitary, thymus, lymph node, heart, lungs, spleen, seminal vesicles, uterus, skin, trachea, liver, stomach, jejunum, kidney, testis, prostate, and ovary were performed | Normal weight gain pattern in all groups; slight increased weight gain in rats receiving MK-7, but increase was not statistically significant. Male and female rats showed significant weight increase at 90 d in all treatment group compared with controls. Average weights of organs in male and female rats were not significantly different from those in controls (liver, thymus, kidney, spleen, testis, seminal vesicles, prostate, and uterus). However, female rats in the 0.5-mg/kg group had a statistically significant decrease in heart weight compared with controls. Liver enzymes (SGPT, SGOT, alkaline phosphatase) and, similarly, serum glucose, total protein, creatinine, and blood urea levels showed no significant changes in any group. Uric acid levels were not changed in females, but in males there was a significant decrease at day 45 in the 0.1-mg/kg group. Conversely, levels in both sexes were increased significantly at day 45 and day 90 in the 0.5-mg/kg and 1.0-mg/kg groups. Blood counts (total WBC count, RBC count, and hematocrit) were significantly decreased at day 90 in both sexes; hemoglobin levels were generally the same except in males on day 45 in the 0.1-mg/kg group (increased), on day 45 in the 1.0-mg/kg group (decreased), and in control females (decreased). Mean corpuscular hemoglobin concentration, corpuscular volume, and RBC distribution width values and clotting time were not affected. Urinalysis showed no significant changes in specific gravity or pH. Histopathological study showed no remarkable changes in organs of control or treated animals except in females, in which proliferation of uterine epithelium was seen at all levels in 1–2 rats, while cytoarchitecture was normal in all other rats. Significant levels at P < 0.05 |
| Pucaj et al (2011)30 | Acute oral toxicity test. MK-7 suspended in sunflower oil was administered to mice by single oral gavage to achieve a dose of 2000 mg/kg body weight | Mice were weighed at days 0, 7, and 14 (termination). Animals were monitored twice daily on the day of dosing and once daily thereafter. Observations included changes in skin, fur, eyes, mucous membranes, and respiratory, circulatory, autonomic, and central nervous systems. Animals were also observed for changes in motor activity and behavior pattern | At limit dose level of 2000 mg/kg, MK-7 did not induce any signs of toxicity in any of the treated mice following dosing or during the 14-d observation period. Body weight gain of treated mice was not adversely affected. Median LD50 was > 2000 mg/kg body weight |
| Pucaj et al (2011)30 | Subchronic oral study. Rats were given MK-7 for 90 d at doses of 0, 2.5, 5.0, and 10 mg/kg body weight per day | Rats were observed for clinical signs and mortality twice daily throughout the study and for reaction to treatment such as changes in skin, fur, eyes, and mucous membranes. Rats were also monitored for changes in respiratory, circulatory, autonomic, and central nervous systems, for changes in somatomotor activity and behavior patterns, and for any other signs of ill health. Terminal body weights were recorded on day 91–92 for main study animals. During the recovery, animal weights were determined. Hematologic and clinical chemistry data of rats were obtained and compared with baseline data | No deaths occurred, and no compound-related toxicity was indicated by clinical observations or by ophthalmology, clinical pathology, gross necropsy, or histopathology. Any statistical significant differences in clinical pathology parameters and/or organ weights noted were considered to be within normal biological variability. Median LD50 = 2000 mg/kg and NOAEL = 10 mg/kg body weight per day |
Abbreviations: LD50, lethal dose 50; NOAEL, no observed adverse effect level; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; RBC, red blood cell; WBC, white blood cell.
Animal acute and subchronic toxicity studies with menaquinone-7 (MK-7)
| Reference . | Study design . | Observations . | Results . |
|---|---|---|---|
| Ravishankar et al (2015)132 | Acute oral toxicity study in rats. MK-7 suspended in a propylene glycol vehicle administered orally by gavage at 0.5 mg/kg, 1.0 mg/kg, 10 mg/kg, or 20 mg/kg. MK-7 or placebo containing propylene glycol only administered once daily for 14 d | Rats were monitored for general behavior, toxic signs and symptoms, or mortality during the experimental period. At end of study, mice were killed and examined for gross necropsy performed in vital organs | No effect of MK-7 on food and water consumption, no physical or behavioral changes, and no mortality observed in any group after 14 d. In the 1-mg/kg group, 2 of 8 animals had mild irritability. No statistically significant difference in body weight gain observed in any group. No adverse effects observed in either sex in any group. All rats survived, with no symptoms of distress or toxic effects. LD50 was > 2000 mg/kg body weight |
| Ravishankar et al (2015)132 | Subchronic oral toxicity study in rats. MK-7 administered orally by gavage at 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg. MK-7 or placebo containing propylene glycol only given once daily for 90 d. MK-7 prepared fresh daily and administered as 1 mL/100 g body weight between 8 am and 9 am | Rats were monitored for changes in behavior, mortality, body weight, and food consumption. Blood was collected on days 15, 45, and 91 (at sacrifice) to determine fasting blood sugar; levels of serum urea, creatinine, uric acid, total cholesterol, triglycerides, total protein, and serum calcium; albumin-to-globulin ratio, liver enzymes SGOT and SGPT, and alkaline phosphatase activity. Other hematologic parameters measured on those days included total WBC count, total lymphocyte count, total monocyte count, total granulocyte count, lymphocyte percentage, monocyte percentage, granulocyte percentage, RBC count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, RBC distribution width, and clotting time. Urine specific gravity and pH were measured at days 15, 45, and 91 (at sacrifice). After 90 d, rats were killed and autopsied, and histological studies of brain, pituitary, thymus, lymph node, heart, lungs, spleen, seminal vesicles, uterus, skin, trachea, liver, stomach, jejunum, kidney, testis, prostate, and ovary were performed | Normal weight gain pattern in all groups; slight increased weight gain in rats receiving MK-7, but increase was not statistically significant. Male and female rats showed significant weight increase at 90 d in all treatment group compared with controls. Average weights of organs in male and female rats were not significantly different from those in controls (liver, thymus, kidney, spleen, testis, seminal vesicles, prostate, and uterus). However, female rats in the 0.5-mg/kg group had a statistically significant decrease in heart weight compared with controls. Liver enzymes (SGPT, SGOT, alkaline phosphatase) and, similarly, serum glucose, total protein, creatinine, and blood urea levels showed no significant changes in any group. Uric acid levels were not changed in females, but in males there was a significant decrease at day 45 in the 0.1-mg/kg group. Conversely, levels in both sexes were increased significantly at day 45 and day 90 in the 0.5-mg/kg and 1.0-mg/kg groups. Blood counts (total WBC count, RBC count, and hematocrit) were significantly decreased at day 90 in both sexes; hemoglobin levels were generally the same except in males on day 45 in the 0.1-mg/kg group (increased), on day 45 in the 1.0-mg/kg group (decreased), and in control females (decreased). Mean corpuscular hemoglobin concentration, corpuscular volume, and RBC distribution width values and clotting time were not affected. Urinalysis showed no significant changes in specific gravity or pH. Histopathological study showed no remarkable changes in organs of control or treated animals except in females, in which proliferation of uterine epithelium was seen at all levels in 1–2 rats, while cytoarchitecture was normal in all other rats. Significant levels at P < 0.05 |
| Pucaj et al (2011)30 | Acute oral toxicity test. MK-7 suspended in sunflower oil was administered to mice by single oral gavage to achieve a dose of 2000 mg/kg body weight | Mice were weighed at days 0, 7, and 14 (termination). Animals were monitored twice daily on the day of dosing and once daily thereafter. Observations included changes in skin, fur, eyes, mucous membranes, and respiratory, circulatory, autonomic, and central nervous systems. Animals were also observed for changes in motor activity and behavior pattern | At limit dose level of 2000 mg/kg, MK-7 did not induce any signs of toxicity in any of the treated mice following dosing or during the 14-d observation period. Body weight gain of treated mice was not adversely affected. Median LD50 was > 2000 mg/kg body weight |
| Pucaj et al (2011)30 | Subchronic oral study. Rats were given MK-7 for 90 d at doses of 0, 2.5, 5.0, and 10 mg/kg body weight per day | Rats were observed for clinical signs and mortality twice daily throughout the study and for reaction to treatment such as changes in skin, fur, eyes, and mucous membranes. Rats were also monitored for changes in respiratory, circulatory, autonomic, and central nervous systems, for changes in somatomotor activity and behavior patterns, and for any other signs of ill health. Terminal body weights were recorded on day 91–92 for main study animals. During the recovery, animal weights were determined. Hematologic and clinical chemistry data of rats were obtained and compared with baseline data | No deaths occurred, and no compound-related toxicity was indicated by clinical observations or by ophthalmology, clinical pathology, gross necropsy, or histopathology. Any statistical significant differences in clinical pathology parameters and/or organ weights noted were considered to be within normal biological variability. Median LD50 = 2000 mg/kg and NOAEL = 10 mg/kg body weight per day |
| Reference . | Study design . | Observations . | Results . |
|---|---|---|---|
| Ravishankar et al (2015)132 | Acute oral toxicity study in rats. MK-7 suspended in a propylene glycol vehicle administered orally by gavage at 0.5 mg/kg, 1.0 mg/kg, 10 mg/kg, or 20 mg/kg. MK-7 or placebo containing propylene glycol only administered once daily for 14 d | Rats were monitored for general behavior, toxic signs and symptoms, or mortality during the experimental period. At end of study, mice were killed and examined for gross necropsy performed in vital organs | No effect of MK-7 on food and water consumption, no physical or behavioral changes, and no mortality observed in any group after 14 d. In the 1-mg/kg group, 2 of 8 animals had mild irritability. No statistically significant difference in body weight gain observed in any group. No adverse effects observed in either sex in any group. All rats survived, with no symptoms of distress or toxic effects. LD50 was > 2000 mg/kg body weight |
| Ravishankar et al (2015)132 | Subchronic oral toxicity study in rats. MK-7 administered orally by gavage at 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg. MK-7 or placebo containing propylene glycol only given once daily for 90 d. MK-7 prepared fresh daily and administered as 1 mL/100 g body weight between 8 am and 9 am | Rats were monitored for changes in behavior, mortality, body weight, and food consumption. Blood was collected on days 15, 45, and 91 (at sacrifice) to determine fasting blood sugar; levels of serum urea, creatinine, uric acid, total cholesterol, triglycerides, total protein, and serum calcium; albumin-to-globulin ratio, liver enzymes SGOT and SGPT, and alkaline phosphatase activity. Other hematologic parameters measured on those days included total WBC count, total lymphocyte count, total monocyte count, total granulocyte count, lymphocyte percentage, monocyte percentage, granulocyte percentage, RBC count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, RBC distribution width, and clotting time. Urine specific gravity and pH were measured at days 15, 45, and 91 (at sacrifice). After 90 d, rats were killed and autopsied, and histological studies of brain, pituitary, thymus, lymph node, heart, lungs, spleen, seminal vesicles, uterus, skin, trachea, liver, stomach, jejunum, kidney, testis, prostate, and ovary were performed | Normal weight gain pattern in all groups; slight increased weight gain in rats receiving MK-7, but increase was not statistically significant. Male and female rats showed significant weight increase at 90 d in all treatment group compared with controls. Average weights of organs in male and female rats were not significantly different from those in controls (liver, thymus, kidney, spleen, testis, seminal vesicles, prostate, and uterus). However, female rats in the 0.5-mg/kg group had a statistically significant decrease in heart weight compared with controls. Liver enzymes (SGPT, SGOT, alkaline phosphatase) and, similarly, serum glucose, total protein, creatinine, and blood urea levels showed no significant changes in any group. Uric acid levels were not changed in females, but in males there was a significant decrease at day 45 in the 0.1-mg/kg group. Conversely, levels in both sexes were increased significantly at day 45 and day 90 in the 0.5-mg/kg and 1.0-mg/kg groups. Blood counts (total WBC count, RBC count, and hematocrit) were significantly decreased at day 90 in both sexes; hemoglobin levels were generally the same except in males on day 45 in the 0.1-mg/kg group (increased), on day 45 in the 1.0-mg/kg group (decreased), and in control females (decreased). Mean corpuscular hemoglobin concentration, corpuscular volume, and RBC distribution width values and clotting time were not affected. Urinalysis showed no significant changes in specific gravity or pH. Histopathological study showed no remarkable changes in organs of control or treated animals except in females, in which proliferation of uterine epithelium was seen at all levels in 1–2 rats, while cytoarchitecture was normal in all other rats. Significant levels at P < 0.05 |
| Pucaj et al (2011)30 | Acute oral toxicity test. MK-7 suspended in sunflower oil was administered to mice by single oral gavage to achieve a dose of 2000 mg/kg body weight | Mice were weighed at days 0, 7, and 14 (termination). Animals were monitored twice daily on the day of dosing and once daily thereafter. Observations included changes in skin, fur, eyes, mucous membranes, and respiratory, circulatory, autonomic, and central nervous systems. Animals were also observed for changes in motor activity and behavior pattern | At limit dose level of 2000 mg/kg, MK-7 did not induce any signs of toxicity in any of the treated mice following dosing or during the 14-d observation period. Body weight gain of treated mice was not adversely affected. Median LD50 was > 2000 mg/kg body weight |
| Pucaj et al (2011)30 | Subchronic oral study. Rats were given MK-7 for 90 d at doses of 0, 2.5, 5.0, and 10 mg/kg body weight per day | Rats were observed for clinical signs and mortality twice daily throughout the study and for reaction to treatment such as changes in skin, fur, eyes, and mucous membranes. Rats were also monitored for changes in respiratory, circulatory, autonomic, and central nervous systems, for changes in somatomotor activity and behavior patterns, and for any other signs of ill health. Terminal body weights were recorded on day 91–92 for main study animals. During the recovery, animal weights were determined. Hematologic and clinical chemistry data of rats were obtained and compared with baseline data | No deaths occurred, and no compound-related toxicity was indicated by clinical observations or by ophthalmology, clinical pathology, gross necropsy, or histopathology. Any statistical significant differences in clinical pathology parameters and/or organ weights noted were considered to be within normal biological variability. Median LD50 = 2000 mg/kg and NOAEL = 10 mg/kg body weight per day |
Abbreviations: LD50, lethal dose 50; NOAEL, no observed adverse effect level; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; RBC, red blood cell; WBC, white blood cell.
Genotoxicity, mutagenicity, and carcinogenicity studies with menaquinone-7 (MK-7)
| Reference . | Test type . | Description . | Dose . | Results . | Conclusions . |
|---|---|---|---|---|---|
| Ravishankar et al (2015)132 | Genotoxicity testing | Frequency of chromosomal aberrations and number of micronuclei and comet assay tests in rats | 0.1 mg/kg or 1.0 mg/kg, given orally once daily for 28 d | Frequency of chromosomal aberrations and number of micronuclei did not differ statistically significantly between group treated with MK-7 and control group. No qualitative or quantitative DNA damage in cells of animals supplemented with MK-7 compared with control animals | MK-7 had no genotoxic effects in rats at intakes of 1.0 mg/kg |
| Ravishankar et al (2015)132 | Mutagenicity testing | Ames text using Salmonella typhimurium strains TA 1535, TA97a, TA98, TA100, and TA102 with or without presence of a metabolic activation system | Test strains treated with 0.02 mg, 0.06 mg, 0.2 mg, 0.6 mg, or 2.0 mg | MK-7–treated group showed no change in microbial growth patterns, with number of revertant colonies indicating nonmutagenicity of the tested preparation | MK-7 was not mutagenic at concentrations ranging from 0.02 mg to 2.0 mg |
| Reference . | Test type . | Description . | Dose . | Results . | Conclusions . |
|---|---|---|---|---|---|
| Ravishankar et al (2015)132 | Genotoxicity testing | Frequency of chromosomal aberrations and number of micronuclei and comet assay tests in rats | 0.1 mg/kg or 1.0 mg/kg, given orally once daily for 28 d | Frequency of chromosomal aberrations and number of micronuclei did not differ statistically significantly between group treated with MK-7 and control group. No qualitative or quantitative DNA damage in cells of animals supplemented with MK-7 compared with control animals | MK-7 had no genotoxic effects in rats at intakes of 1.0 mg/kg |
| Ravishankar et al (2015)132 | Mutagenicity testing | Ames text using Salmonella typhimurium strains TA 1535, TA97a, TA98, TA100, and TA102 with or without presence of a metabolic activation system | Test strains treated with 0.02 mg, 0.06 mg, 0.2 mg, 0.6 mg, or 2.0 mg | MK-7–treated group showed no change in microbial growth patterns, with number of revertant colonies indicating nonmutagenicity of the tested preparation | MK-7 was not mutagenic at concentrations ranging from 0.02 mg to 2.0 mg |
Genotoxicity, mutagenicity, and carcinogenicity studies with menaquinone-7 (MK-7)
| Reference . | Test type . | Description . | Dose . | Results . | Conclusions . |
|---|---|---|---|---|---|
| Ravishankar et al (2015)132 | Genotoxicity testing | Frequency of chromosomal aberrations and number of micronuclei and comet assay tests in rats | 0.1 mg/kg or 1.0 mg/kg, given orally once daily for 28 d | Frequency of chromosomal aberrations and number of micronuclei did not differ statistically significantly between group treated with MK-7 and control group. No qualitative or quantitative DNA damage in cells of animals supplemented with MK-7 compared with control animals | MK-7 had no genotoxic effects in rats at intakes of 1.0 mg/kg |
| Ravishankar et al (2015)132 | Mutagenicity testing | Ames text using Salmonella typhimurium strains TA 1535, TA97a, TA98, TA100, and TA102 with or without presence of a metabolic activation system | Test strains treated with 0.02 mg, 0.06 mg, 0.2 mg, 0.6 mg, or 2.0 mg | MK-7–treated group showed no change in microbial growth patterns, with number of revertant colonies indicating nonmutagenicity of the tested preparation | MK-7 was not mutagenic at concentrations ranging from 0.02 mg to 2.0 mg |
| Reference . | Test type . | Description . | Dose . | Results . | Conclusions . |
|---|---|---|---|---|---|
| Ravishankar et al (2015)132 | Genotoxicity testing | Frequency of chromosomal aberrations and number of micronuclei and comet assay tests in rats | 0.1 mg/kg or 1.0 mg/kg, given orally once daily for 28 d | Frequency of chromosomal aberrations and number of micronuclei did not differ statistically significantly between group treated with MK-7 and control group. No qualitative or quantitative DNA damage in cells of animals supplemented with MK-7 compared with control animals | MK-7 had no genotoxic effects in rats at intakes of 1.0 mg/kg |
| Ravishankar et al (2015)132 | Mutagenicity testing | Ames text using Salmonella typhimurium strains TA 1535, TA97a, TA98, TA100, and TA102 with or without presence of a metabolic activation system | Test strains treated with 0.02 mg, 0.06 mg, 0.2 mg, 0.6 mg, or 2.0 mg | MK-7–treated group showed no change in microbial growth patterns, with number of revertant colonies indicating nonmutagenicity of the tested preparation | MK-7 was not mutagenic at concentrations ranging from 0.02 mg to 2.0 mg |
Safety margin
In a 90-day subchronic toxicity study of MK-7 at oral doses of up to 1 mg/kg/d in Wistar albino rats, no significant dose-dependent adverse effects were observed.132 On the basis of a subchronic toxicity study of MK-7 in Sprague-Dawley rats, Pucaj et al30 set a NOAEL of 10 mg/kg/d. This rat NOAEL value would be converted to the human equivalent dose133 as follows: 10 mg/kg/d × 0.162 (body surface area conversion factor) = 1.62 mg/kg/d.
There are no reliable dietary intake data for MK-7. Using vitamin K1 dietary intake as a proxy, however, the NHANES 2011–201275 analysis gives a mean intake level of 139 µg/d in adult males (1.99 µg/kg/d) and of 120.7 µg/d (2.1 µg/kg/d) in adult females. Turning back to the NHANES III (1988–1994)9 data for the 95th percentile of intake, the maximum value reported was 223 µg/d in males 51–70 years of age, which is equivalent to 3 µg/kg/d.
Comparing the human equivalent dose derived from the Pucaj et al30 data with the maximum 95th percentile of intake, the safety margin would be 1620 ÷ 3 = 540. Comparing this human equivalent dose with current mean intake levels for men and women would yield a safety margin of 1620 ÷ 2 = 810. The lower safety margin of 540 is much higher than the 100 that is typically applied as an uncertainty factor to animal doses to convert the NOAEL into an acceptable daily intake in humans. To compare this calculation with a previous safety margin assessment, the 2008 EFSA Panel calculated a safety margin from the highest 97.5th percentile intake estimate for children (5.4 µg/kg/d) and a LOAEL (20 mg/kg/d) from a rat study as 3700. As the intake estimates are highly conservative, they also concluded that the use of MK-7 at the proposed levels of use presents no safety concerns.24
CONCLUSION
Menaquinone-7 is a form of vitamin K2 biosynthesized by bacteria. It occurs naturally in some meat (<0.5 µg/100 g), dairy (0.1–65 µg/100 g), and fermented foods (up to 1000 µg/100 g in natto). However, the amount of MK-7 absorbed from the intestinal microflora is unknown compared with that absorbed from dietary intake. Menaquinone-7 is also available to consumers as a synthetic or fermentation-derived ingredient of more than 200 marketed dietary supplements in the United States and more than 500 natural health products in Canada. Product labels typically recommend an intake of approximately 50 µg/d but may recommend up to 600 µg/d in the United States and up to 120 µg/d in Canada.
Menaquinone-7 intake contributes probably less than 5% of the dietary intake of vitamin K, which is primarily as vitamin K1 in Western countries. Among adults, the mean intake of vitamin K1 from food and beverages is generally higher than the IOM Adequate Intake (116% in men and 134% in women). The mean intake of vitamin K1 from supplements adds 32 µg/d in men and 35 µg/d in women, bringing users of supplements to 152% (men) and 182% (women) of their Adequate Intake values, respectively. Both MK-7 and vitamin K1 can be converted by the body into the active form, MK-4, but the length of the polyisoprenoid side chain also affects the pharmacokinetics and bioactivity of menaquinone vitamers. Vitamin K plays important nutritional roles in maintaining normal blood coagulation, bone mineralization, soft tissue physiology, and neurological development.
The lack of adverse effects of MK-7 in healthy humans precluded the IOM, the European Commission, the UK EVM, and the WHO/FAO from setting a Tolerable Upper Intake Level for any form of vitamin K, as levels of intake in excess of the Adequate Intake, even with additional intake of MK-7 from foods and supplements, cannot be interpreted as presenting a potential risk to health. The UK EVM recommended a Guidance Level of 1000 µg/d and the Council for Responsible Nutrition set a Tolerable Upper Intake Level for supplements of 10 000 µg/d.
No reports were found of human clinical trials that were designed and statistically powered to evaluate the safety of MK-7. Studies of MK-7 in animals for acute, subchronic, reproductive and developmental toxicity, and genotoxicity and in vitro studies for mutagenicity and carcinogenicity showed no significant risks. On the basis of a NOAEL from a subchronic toxicity study of MK-7 in rats by Pucaj et al,30 conversion of this value to the human equivalent dose and comparison with the NHANES III maximum 95th percentile of intake yielded a safety margin of 540. The EFSA24 used both a LOAEL based on a study of MK-4 in rats and the 97.5th percentile of vitamin K intake in children to derive a safety margin of 3700. As these intake estimates are highly conservative, such large safety margins indicate that the use of MK-7 at levels of up to 6 µg/kg/d in the healthy population is unlikely to pose any risk to health.
Since MK-7 is made from soy protein, and both the FDA and Health Canada recognize soy as a major food allergen despite the likely very low levels of protein carryover from fermentation, labeling of MK-7 products as being prepared from soy is recommended so that consumers can make informed choices.
There is a risk for interaction between MK-7 and anticoagulant drugs, but patients on anticoagulation therapy receive advice from their physician and pharmacist specifically warning about both the need to maintain a stable dietary intake of vitamin K and the risk of an interaction with vitamin K supplements. Menaquinone-7 is already listed as a form of vitamin K on product labels.
The clinical and nonclinical data reviewed here, together with the findings of reviews conducted by reputable bodies such as the EFSA, the UK EVM, the IOM, and WHO, support the conclusion that MK-7, when ingested as a dietary supplement at levels typically recommended, is not likely to be associated with any serious risk to individual or public health that is not already addressed by current practices regarding the marketing and use of dietary supplements containing vitamin K in its various forms.
Acknowledgments
The authors would like to acknowledge with great appreciation the advice and suggestions provided by the members of the 2010–2015 cycle of the USP Dietary Supplements Admission Evaluation Subcommittee during the development of this manuscript. The authors thank colleagues at USP: Nandakumara Sarma, PhD, and Gabriel Giancaspro, PhD, for critically reviewing the manuscript; Doug Podolky, for editorial assistance; and Carlos Celestino, JD, for legal counsel. The authors would also like to acknowledge Brittany Poe (Procter & Gamble Co.) for her assistance in preparing this paper for publication.
Funding/support. Funding for this review was provided by the United States Pharmacopeial Convention.
Declaration of interest. The authors have no relevant interests to declare.