-
PDF
- Split View
-
Views
-
Cite
Cite
Elżbieta Żbikowska, Paola Lombardo, Janusz Żbikowski, Grażyna Jabłońska, Anna Marszewska, Anna Cichy, Ketoprofen-induced inhibition of symptoms of behavioural fever observed in wintering Planorbarius corneus (L.) (Gastropoda: Planorbidae), Journal of Molluscan Studies, Volume 83, Issue 4, November 2017, Pages 434–439, https://doi.org/10.1093/mollus/eyx026
Close - Share Icon Share
Abstract
Research on behavioural fever in Planorbarius corneus was undertaken using a longitudinal thermal gradient. Before the experiment, snails were acclimated at 19 °C. Following injections of lipopolysaccharide (LPS, a pyrogenic agent), ketoprofen (an antipyretic) or saline solution (control), thermal behaviour of the animals was automatically recorded for 48 h. The results demonstrated that LPS-induced symptoms of behavioural fever were inhibited by ketoprofen. Additionally, it was observed that snails which, before the injection of LPS, were preinjected with ketoprofen showed symptoms of behavioural fever with a 20-h delay. This result shows the inhibitory effect of ketoprofen on the occurrence of behavioural fever symptoms in P. corneus. It may suggest some similarity in mechanism of enhanced thermal preference in snails to vertebrate fever—both effectively inhibited by aspirin-like anti-inflammatory compounds.
INTRODUCTION
The phenomenon of fever in response to the injection of pyrogens is appropriate not only to endothermic animals, but has been widely described in many ectotherm groups as a ‘behavioural fever’ (Reynolds, Casterlin & Covert, 1976; Kluger, 1986; Cooper, 1995; Blanford, Thomas & Langewald, 1998; Cabanac & Bernieri, 2000). Boltaña et al. (2013) pointed out the therapeutic role of thermal behaviour of fish during viral infection. Monagas & Gatten (1983) observed the symptoms of this kind of fever in turtles after injection of live bacteria and Woodhams, Alford & Marantelli (2003) emphasized that the choice of warmer places in the environment by amphibians infected with pathogenic fungi affected the regression of infection. The phenomenon of behavioural-raising of body temperature in ectotherms is still not well known and much of our understanding is based on observations of warm-blooded animals (see review by Demas & Nelson, 2012). An additional challenge in studying the possible therapeutic role of thermal behaviour in ectotherms is the use of invertebrate models—mainly arthropods or molluscs which, in contrast to vertebrate ectotherms, do not possess structures homologous to the organs of endothermic vertebrates.
Insects (Hunt & Charnley, 2011; De Roode & Lefevre, 2012; Anderson, Blanford & Thomas, 2013) and, much less frequently, gastropod molluscs (Cabanac & Rossetti, 1987; Żbikowska et al., 2013a,b) are commonly used in studies of behavioural fever in invertebrate ectotherms. Both the lack of anatomically defined thermosensitive organs in molluscs and the claim, widely quoted in the past, about the phylogeny of fever processes (Cabanac & Rossetti, 1987), have for a long time hindered the studies on the therapeutic significance of snail thermal preferences. In a breakthrough study, Lefcort & Bayne (1991) found that Biomphalaria glabrata individuals infected with Schistosoma mansoni preferred a lower ambient temperature than control individuals. However, these authors found no symptoms of behavioural fever and abandoned further examination. In fact, other observations have described symptoms of behavioural anapyrexia (i.e. reverse fever) in studies on pulmonate gastropods infected with Digenea larvae (Żbikowska, 2005, 2011; Żbikowska & Cichy, 2012; Żbikowska & Żbikowski, 2015). Subsequent studies of thermal behaviour of snails treated with viral (poly I:C), bacterial (lipopolysaccharide, LPS) or fungal (zymosan) pyrogens (Żbikowska et al., 2013b) have shown that exogenous pyrogenic factors may cause changes in preferences of Planorbarius corneus (L.) for higher temperatures. However, the symptoms of behavioural fever in response to pyrogen injection is not sufficient evidence to prove the protective nature of the response.
The cyclooxygenase (COX) known as prostaglandin (PG) synthase is involved in the mechanism of fever in vertebrates (Zhang et al., 2003; Ricciotti & FitzGerald, 2011). Antipyretic agents commonly used in vertebrates are nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit COX (Baek et al., 2002). Given that multiple forms of this enzyme have been found in vertebrates and invertebrates, including molluscs (Kawamura et al., 2014), we hypothesized that the LPS-dependent change in thermal behaviour of P. corneus could be inhibited by nonsteroidal anti-inflammatory agents. A positive verification of this hypothesis would be preliminary evidence for the participation of COX-like enzymes in generating symptoms of behavioural fever in this snail species.
MATERIAL AND METHODS
Pyrogenic and antipyretic agents
Ketoprofen, or (RS)-2-(3-benzoylphenyl)-propionic acid (C16H14O3), is an NSAID with analgesic and antipyretic effects. Ketoprofen is generally prescribed in human and veterinary medicine for fever, arthritis-related inflammatory pains or severe neurological and musculoskeletal pain (e.g. Glew et al., 1997; Mazières et al., 2005; Forney, 2007). It is sold as a topical cutaneous cream or in tablet or capsule form, as an over-the-counter or prescription medication.
LPS Escherichia coli 0111:B4 is an LPS extracted from E. coli serotype 0111:B4 and purified by gel filtration (Sigma Chemicals). This LPS serotype has been used to stimulate B-cells and induce NOS in human hepatocytes. It is a known pyrogenic substance that elicits strong immune responses in animals (Migale et al., 2015).
Snails
Specimens of Planorbarius corneus was collected at the end of the active season (September/October 2014) in Gopło Lake (central Poland). Water conditions were: temperature 12.8 °C; pH 8.5; dissolved oxygen saturation 133%; conductivity 603 μS cm−1; total phosphorus concentration 0.193 mg l−1; calcium content 74.9 mg l−1. Over 250 adult specimens, with shell diameter in the range of 30–35 mm, were sampled. Parasitic incidence for the Gopło Lake population of P. corneus has been consistently low for the past few years (Żbikowska & Nowak, 2009; Cichy, Faltynkova & Żbikowska, 2011). However, we checked all collected snails for the presence of Digenea larvae by observing emerging cercariae. Only snails not shedding cercariae were initially considered to be noninfected. After experimentation all animals were dissected and the lack of trematode larvae was verified. Following Żbikowska's (2011) protocol, all animals to be used in thermo-behavioural experiments were kept in containers with spring water under artificial winter conditions, i.e. in a refrigerator (temperature: 4–6 °C) for 12 weeks. Such spring water was chemically suitable for P. corneus based on preliminary observations of sustained growth and reproduction of captive specimens. Before placing them into a thermal gradient, every snail was acclimated to 19 °C (i.e. within the approx. 0.15–25 °C optimum range for growth and survivorship; Costil, 1994) in a natural photoperiod corresponding to late winter (in a cooled incubator, Sanyo MIR-153) for at least 3 weeks. Based on preliminary observations, such treatment was required to stimulate wintering snails to move in a thermal gradient. The spring water in containers was changed every week. Animals were fed with commercially available lettuce. During the study none of the snails produced egg cocoons, in accordance with the known cessation of reproduction in the winter (Costil & Daguzan, 1995).
Treatment of snails before thermal experiments
Snails were divided into five groups, three treatments and two controls. The first experimental group of individuals (LPS treatment) were injected with 100 μl E. coli LPS (0111:B4, Sigma Chemicals) at the nontoxic dose of 10 μg g−1 (Żbikowska et al., 2013b). A second experimental group (K + LPS treatment) was pretreated with 100 μl volume containing 100 μg g−1 of ketoprofen 4 h before LPS injection, and the third group (LPS + K treatment) was pretreated with LPS 4 h before ketoprofen injection. Before injection, the stock solutions of LPS (2 mg ml−1) or ketoprofen (5 mg ml−1) were diluted in a sterile saline (0.6% NaCl) solution to the desired concentration. All doses were calculated according to wet body mass of snails (without shell). For this purpose, additional snails from lake Gopło, with shells of the same size as experimental individuals, were used. Snails treated with a second injection 4 h after first one were kept in an incubator at 19 °C. The 4-h interval between injection of LPS and ketoprofen doses corresponds approximately to the observed latency period before the appearance of behavioural fever in P. corneus individuals after pyrogen injection (Żbikowska et al., 2013b).
Two control groups of snails were set up: the first control group (NaCl treatment) was injected with 100 μl of 0.6% NaCl solution, and the second control group (K treatment) was treated with 100 μl of only the ketoprofen solution.
Liquids were injected into the dorsal surface of the foot using a sterile single-use 1-ml syringe. Muscle contraction of the foot prevented leakage of fluids. All snails were injected at noon without anaesthesia.
Observations in a thermal gradient
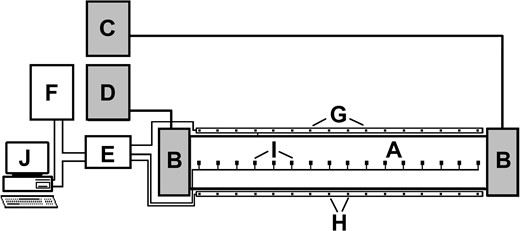
At the same time, a snail from each of the five groups was placed individually in five separate rectangular thermal gradient chambers, constructed for studies on the thermal behaviour of aquatic snails (Żbikowska & Cichy, 2012; Żbikowska et al., 2013b). Each such chamber (Fig. 1) included a 130-cm long ‘channel’ containing 0.9 l spring water with an end-to-end temperature range of 4 to 38 °C, thus including the optimal c. 10–25 °C temperature range for P. corneus (e.g. Costil, 1994). The temperature gradient was generated and controlled by a PolyScience ultrathermostat and cryostat (Fig. 1). Each experimental snail was introduced to a thermal gradient chamber at the point where the temperature was 19 °C, thus avoiding any thermal shock to the snails. Experimental trials were carried out in an air-conditioned room at 19–20 °C. Each experimental trial lasted 48 h, with the position of snails along the thermal gradient automatically recorded via infra-red beam detection and computed at 3-min intervals using a customized data-acquisition computer program GRAD (Żbikowska et al., 2013b). Each snail was used only once.
Experimental setup for recording thermal behaviour of snails. Abbreviations: A, thermal gradient chamber (chamber length 130 cm, water depth 0.5 cm); B, fluid chambers; C, thermostat; D, cryostat; E, electronic switch of thermocouples; F, scanner; G, transmitters of infra-red radiation; H, receivers of infra-red radiation; I, thermocouples; J, computer.
Statistical analyses
Snail positions along the thermal gradient (as temperature positions) were pooled into 1-h, 24-h or 48-h averages. Each experimental group consisted of 30 snails. Thermal preference data (first 24-h and second 24-h averages) were analysed with a two-way ANOVA, followed by HSD Tukey tests. A repeated-measures ANOVA followed by LSD Fisher test and Bonferroni corrections was used to analyse 1-h temperature averages. Normality and homoscedasticity of the data were confirmed with Shapiro-Wilk and Levene tests, respectively. For statistical analyses XLSTAT software was used.
RESULTS
Both treatment and time had an impact on thermal preferences of snails (F4,150 = 74.18, P ≪ 0.0001). The Planorbarius corneus individuals from the NaCl and K control groups chose a temperature near the acclimation point of 19 °C during the 48 h duration of the experiment. A slightly lower temperature was chosen during the second 24-h trial period by K snails (i.e. that had been injected with ketoprofen solution), but the difference was not statistically significant from the NaCl control group (P = 0.367; Table 1).
Average values of thermal preferences of Planorbarius corneus individuals under the thermal gradient.
| Experimental group . | No. of replicate snails . | Average temperature (°C) for first 24 h of experiment (±SD) . | Average temperature (°C) for second 24 h of experiment (±SD) . |
|---|---|---|---|
| NaCl (control animals injected with 100 μl of 0.6% saline) | 30 | 19.9 (±2.3)a | 19.8 (±2.6)a |
| K (control animals injected with 100 μl of 100 μg g−1 ketoprofen) | 30 | 18.5 (±1.7)a | 17.8 (±1.6)a |
| LPS (experimental animals injected with 100 μl of 10 μg g−1 LPS) | 30 | 27.1 (±2.2)b | 28.9 (±3.1)c |
| LPS + K (experimental animals with double injection: 100 μl LPS, then 100 μl ketoprofen) | 30 | 18.6 (±0.5)a | 18.5 (±0.7)a |
| K + LPS (experimental animals with double injection: 100 μl ketoprofen then 100 μl LPS) | 30 | 19.9 (±0.8)a | 25.2 (±1.9)d |
| Experimental group . | No. of replicate snails . | Average temperature (°C) for first 24 h of experiment (±SD) . | Average temperature (°C) for second 24 h of experiment (±SD) . |
|---|---|---|---|
| NaCl (control animals injected with 100 μl of 0.6% saline) | 30 | 19.9 (±2.3)a | 19.8 (±2.6)a |
| K (control animals injected with 100 μl of 100 μg g−1 ketoprofen) | 30 | 18.5 (±1.7)a | 17.8 (±1.6)a |
| LPS (experimental animals injected with 100 μl of 10 μg g−1 LPS) | 30 | 27.1 (±2.2)b | 28.9 (±3.1)c |
| LPS + K (experimental animals with double injection: 100 μl LPS, then 100 μl ketoprofen) | 30 | 18.6 (±0.5)a | 18.5 (±0.7)a |
| K + LPS (experimental animals with double injection: 100 μl ketoprofen then 100 μl LPS) | 30 | 19.9 (±0.8)a | 25.2 (±1.9)d |
Different letters (a–d) indicate statistically significant post-hoc Tukey tests (P < 0.001).
Average values of thermal preferences of Planorbarius corneus individuals under the thermal gradient.
| Experimental group . | No. of replicate snails . | Average temperature (°C) for first 24 h of experiment (±SD) . | Average temperature (°C) for second 24 h of experiment (±SD) . |
|---|---|---|---|
| NaCl (control animals injected with 100 μl of 0.6% saline) | 30 | 19.9 (±2.3)a | 19.8 (±2.6)a |
| K (control animals injected with 100 μl of 100 μg g−1 ketoprofen) | 30 | 18.5 (±1.7)a | 17.8 (±1.6)a |
| LPS (experimental animals injected with 100 μl of 10 μg g−1 LPS) | 30 | 27.1 (±2.2)b | 28.9 (±3.1)c |
| LPS + K (experimental animals with double injection: 100 μl LPS, then 100 μl ketoprofen) | 30 | 18.6 (±0.5)a | 18.5 (±0.7)a |
| K + LPS (experimental animals with double injection: 100 μl ketoprofen then 100 μl LPS) | 30 | 19.9 (±0.8)a | 25.2 (±1.9)d |
| Experimental group . | No. of replicate snails . | Average temperature (°C) for first 24 h of experiment (±SD) . | Average temperature (°C) for second 24 h of experiment (±SD) . |
|---|---|---|---|
| NaCl (control animals injected with 100 μl of 0.6% saline) | 30 | 19.9 (±2.3)a | 19.8 (±2.6)a |
| K (control animals injected with 100 μl of 100 μg g−1 ketoprofen) | 30 | 18.5 (±1.7)a | 17.8 (±1.6)a |
| LPS (experimental animals injected with 100 μl of 10 μg g−1 LPS) | 30 | 27.1 (±2.2)b | 28.9 (±3.1)c |
| LPS + K (experimental animals with double injection: 100 μl LPS, then 100 μl ketoprofen) | 30 | 18.6 (±0.5)a | 18.5 (±0.7)a |
| K + LPS (experimental animals with double injection: 100 μl ketoprofen then 100 μl LPS) | 30 | 19.9 (±0.8)a | 25.2 (±1.9)d |
Different letters (a–d) indicate statistically significant post-hoc Tukey tests (P < 0.001).
The preferences of experimental individuals injected with LPS, LPS + K or K + LPS were, however, divergent. The snails injected with LPS at the nontoxic dose significantly (P ≪ 0.0001) chose a higher temperature than both control groups during both the first and second 24 h of the experiment. There was no statistical difference (P = 0.471) in preferred average temperature between control animals and LPS + K snails (i.e. snails pretreated with a double injection of LPS and ketoprofen) (Table 1). All these groups chose to stay near the acclimation temperature during the first and second 24 h of study. Snails pretreated with ketoprofen and then injected with LPS (i.e. K + LPS snails) subsequently preferred statistically higher temperatures than control snails during the second 24 h of observation (Table 1). Snails injected only with the pyrogenic LPS agent not only exhibited a statistically significant preference for higher temperatures in the first 24 h of observations, but also preferred the highest temperature (Table 1), possibly even slightly higher than the optimum temperature range of 10–25 °C reported for P. corneus (Costil, 1994).
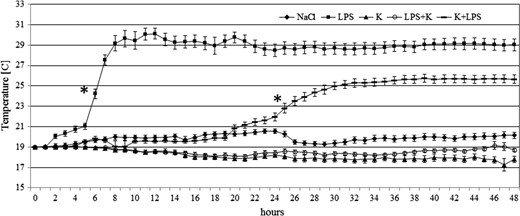
The patterns of the thermo-behavioural response of the two experimental groups LPS and K + LPS were not identical. ANOVA analysis for systems with repeated measurements revealed a significant treatment × time interaction (F188,7050 = 48.49, P < 0.001). The snails which had been injected with 10 μg g−1 of LPS chose a significantly higher temperature than both control groups by the sixth hour of study (P < 0.001) and the individuals pretreated with ketoprofen followed by LPS (K + LPS) chose a higher temperature than control animals at the 26th hour (P < 0.001) of observation (Fig. 2). The average temperatures calculated for two ‘feverish’ groups of snails after the latency period of 5 h or 25 h respectively were: 28.9 ± 2.7 °C (LPS) and 25.3 ± 1.9 °C (K + LPS). Feverish animals stayed at the warm end of the thermal gradient during the rest of the experimental time (Fig. 2).
Thermal preferences (i.e. snail location along the thermal chamber expressed as water temperature) of Planorbarius corneus individuals during the 48-h experiment in a thermal gradient, after treatment with pyrogenic (lipopolysaccharide, LPS) and/or antipyretic (ketoprofen, K) compounds. Each data point is an average value ± SD from 30 snail replicates. Asterisk indicates the experimental time (h) when thermal preference of experimental groups became significantly different from that of NaCl control group (according to Bonferroni-corrected LSD Fisher test following a significant repeated-measures ANOVA).
DISCUSSION
The results indicate that LPS extracted from Escherichia coli (0111:B4) causes behavioural fever symptoms in Planorbarius corneus. The movement of LPS-treated snails to microenvironments with higher temperatures has been indicated in a previous paper in which we presented observations of P. corneus over 24 h in a thermal gradient (Żbikowska et al., 2013b). LPS has the same pyrogenic properties for P. corneus as for fish (Cabanac & Laberge, 1998), amphibian tadpoles (Llewellyn et al., 2011) and reptiles (Merchant et al., 2008). The similarity between the LPS-induced snail behaviour and the behavioural fever of ectothermic vertebrates was evident not only in the preferences for high temperatures 24 and 48 h after LPS injection, but also in the presence of a latency period prior to the feverish reaction (Fig. 2). The latency duration that we found for P. corneus is much longer than that recorded in ectothermic vertebrates (Myhre, Cabanac & Myhre, 1977), but this may be due to differences in the metabolic rates of the animals or in their perception sensitivity to thermal stimuli (Callow, 1977).
Of particular interest is the finding that ketoprofen injected 4 h after LPS dose inhibited the symptoms of behavioural fever in P. corneus (Fig. 2). The LPS + K snails exhibited no preference for a higher temperature compared with the control animals throughout the 48 h of observation (Table 1). Bicego et al. (2002) observed a similar inhibitory effect of anti-inflammatory agents on behavioural fever in amphibians and suggested possible similarities between the mechanism of behavioural fever of vertebrate ectotherms and endotherm fever. In endotherms, cytokines are involved in the chain reaction leading to the synthesis of PGs involved in set-point regulation (Veale, Cooper & Pittman, 1977; Ricciotti & FitzGerald, 2011; Johnson-Rowsey, 2013). Unfortunately, the absence of homologous anatomical structures does not allow for a generalization of fever mechanism(s) across animal phyla, but it does not exclude some metabolic similarity in the mode of raising of body temperature in vertebrates and invertebrates. The presence of cytokines or cytokine-like substances in invertebrates has been documented (Beschin et al., 2001; Malagoli, 2010). The similarity of defence processes involving cytokine-like substances in molluscs and vertebrates was highlighted by Humphries & Yoshino (2003), although they did not demonstrate the role of these substances in the febrile process of invertebrates. We show that the NSAID ketoprofen can inhibit LPS-induced behavioural fever in P. corneus. The effect of NSAIDs on fever suppression is a well-known mechanism in vertebrates and involves antipyretic COX inhibition (Arnoff & Neilson, 2001). According to Kawamura et al. (2014), multiple COX isoforms have been found in vertebrates and invertebrates (including molluscs). The effect of ketoprofen on LPS-dependent behavioural fever in P. corneus (Table 1; Fig. 2) suggests a possible similarity of metabolic processes across multiple animal phyla, although many questions remain unanswered.
In our experiment, snails belonging to the group LPS + K not only did not show feverish symptoms, but additionally were characterized by low mobility in the thermal gradient. This could be an effect of additional injuries caused by a double injection. In previous studies conducted in our laboratory (Żbikowska, 2006) snails that had been repeatedly punctured with a needle or by parasitic larvae, remained motionless in the places of reduced temperature. However, such an explanation cannot explain the behaviour of individuals belonging to K + LPS group, also with double injections, which actively moved to slightly warmer waters 24 h after injection (Fig. 2). The comparison of the results from the two experimental groups with double injection (K + LPS and LPS + K, respectively) suggests that injury was not a crucial factor affecting snail behaviour in the thermal gradient. By analogy to the aforementioned processes identified in vertebrates, the ketoprofen injection 4 h after LPS treatment could have inhibited the motility of snails, and/or a COX-like dependent metabolic pathway could have led to behavioural fever. A slightly different process took place in the case of individuals in the K + LPS group. Here, a double injection, and/or ketoprofen influence, possibly caused reduced motility of snails initially, but perhaps there was no inhibition of a COX-like trail of fever induction. The LPS-dependent process took place several hours after ketoprofen treatment (Fig. 2). According to Alkatheeri, Wasfi & Lambert (1999) and Hutchinson et al. (2014), the apparent half-life values of different NSAIDs in vertebrate animals range from a few to several hours. How long ketoprofen remains active in the snail body is not known. However, if the proposed sequence of events can be accepted, it can be considered that nearly 30 h after ketoprofen injection the snails develop the LPS-derived symptoms of behavioural fever (Fig. 2).
Our data suggest that ketoprofen does not cause major behavioural responses of P. corneus, which may be relatively safe from exposure to ketoprofen in the environment, in contrast with the lethal effect observed on non-target vertebrates (Naidoo et al., 2009). Little is known about the potential biological impact of this and other aspirin-like pharmaceutical residues in the aquatic environment where they can be released via treated wastewater (Chan et al., 2014; Kostich, Batt & Lazorchak, 2014; Batt et al., 2016). Despite the potentially low impact of ketoprofen on P. corneus behaviour, the slight (but, in our study, nonsignificant) preference for slightly colder water by K-treated snails (Fig. 2; Table 1), although unlikely to cause any marked change in snail growth and reproduction (Costil, 1994; Costil & Daguzan, 1995), should be examined in future studies on the ability of infected P. corneus to adapt to the global warming trend in climate (Angilletta, 2006; Rempfer et al., 2010).
In conclusion, it should be emphasized that the results of the experiment do not clearly indicate a similarity of the mechanism of LPS-induced thermal behaviour of snails to the fever of warm-blooded animals, but they can be considered as a basis for research on the effects of aspirin-like anti-inflammatory compounds on the physiology of molluscs. Investigations on the possible ecological effects of pyrogenic and antipyretic residues on aquatic snails and other freshwater biota are also needed.
ACKNOWLEDGEMENTS
We are deeply indebted to Dr Sylwia Wrotek to her help in preparation of injection solutions. This work was supported by statutory research funding from the Faculty of Biology and Environmental Protection of Nicolaus Copernicus University.