-
PDF
- Split View
-
Views
-
Cite
Cite
Yu Tang, Pan Fu, Ying Zhou, Yingzhou Xie, Jialin Jin, Bei Wang, Lianhua Yu, Yunkun Huang, Gang Li, Meng Li, Wei Liang, Hong-Yu Ou, Xiaofei Jiang, Absence of the type I-E CRISPR-Cas system in Klebsiella pneumoniae clonal complex 258 is associated with dissemination of IncF epidemic resistance plasmids in this clonal complex, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 4, April 2020, Pages 890–895, https://doi.org/10.1093/jac/dkz538
Close - Share Icon Share
Abstract
The pandemics caused by MDR Klebsiella pneumoniae are mostly due to the global dissemination of high-risk clonal complex 258 (CC258) and related IncF epidemic plasmids. However, the factors leading to the epidemiological advantages of CC258–IncF linkage remain obscure. The Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) and CRISPR-associated protein (CRISPR-Cas) systems, providing adaptive immunity against invading DNA, play an important role in the interactions between plasmids and hosts.
To investigate the relationship between CRISPR-Cas systems and the high-risk linkage CC258–IncF.
CRISPR-Cas loci were detected among 381 collected K. pneumoniae clinical isolates and 207 K. pneumoniae complete genomes available in GenBank. MLST was used to determine the genetic relatedness of these isolates. Nucleotide BLAST was used to search for protospacers on K. pneumoniae plasmids.
We observed an epidemic correlation between CRISPR-Cas loci, CC258 and IncF plasmids. Interestingly, most type I-E CRISPR-Cas systems identified carried spacers matching the backbone regions of IncF plasmids.
Our results suggest that the absence of type I-E CRISPR-Cas systems in K. pneumoniae CC258 is strongly associated with the dissemination of IncF epidemic plasmids, contributing to the global success of the international high-risk linkage CC258–IncF. Our findings provide new information regarding the dissemination and evolution of the high-risk linkage of K. pneumoniae CC258–IncF and pave the way for new strategies to address the problem of antibiotic resistance.
Introduction
Klebsiella pneumoniae is a major cause of hospital-acquired infections such as pneumonia, bloodstream-associated infections and urinary tract infections.1,K. pneumoniae clonal complex 258 (CC258), mainly consisting of ST258, ST11, ST340 and ST512, has spread extensively throughout the world. This international high-risk CC is notorious for its contribution to the spread of multiple β-lactamases, with KPC being predominant and also including CTX-M, OXA and NDM β-lactamases.1–3
Plasmids belonging to incompatibility group F (IncF) are mostly narrow-host-range plasmids and tend to be restricted to species. IncF plasmids are also termed epidemic resistance plasmids due to their propensity to acquire resistance genes and rapidly disseminate among Enterobacteriaceae, especially within species.4 The global dissemination of K. pneumoniae CC258 is strongly related to IncF epidemic plasmids (specifically IncF with certain β-lactamases).1,5,6 Although the reasons behind this phenomenon are unclear, the ability of the CC258–IncF linkage to spread swiftly is beyond dispute.
The Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) and CRISPR-associated protein (CRISPR-Cas) systems provide adaptive immunity against mobile genetic elements (MGEs), particularly viruses and plasmids, by sequence-specific targeting of foreign DNA.5 They play an important role in the interactions between plasmids and hosts.6,7 A CRISPR-Cas locus generally consists of an operon of CRISPR-associated (cas) genes and a CRISPR array composed of DRs (25–35 bp in size) that are separated by variable DNA sequences (known as ‘spacers’, typically 30–40 bp).6 CRISPR-Cas immunity involves three distinct mechanistic stages: spacer acquisition, pre-CRISPR RNA (pre-crRNA) processing and interference.5 The new classification of CRISPR-Cas encompasses two classes, five types and 16 subtypes.8 Type I-E CRISPR-Cas systems are known in K. pneumoniae and they commonly have eight cas genes and either one or two flanking CRISPR arrays.9,10 On the basis of different layouts, they were further classified and designated as type I-E and type I-E* (Figure S1, available as Supplementary data at JAC Online).10 Type I-E (in the cysH-iap region) are canonical type I-E CRISPR-Cas systems, containing consistent DRs (29 bp in length) and a cas operon with only one CRISPR array (designated as CRISPR1) downstream of the cas genes. Compared with canonical type I-E, type I-E* (in the ABC transport system-glyoxalase region) is variable, occasionally with a transposase gene integrated into the cas operon, and harbours two CRISPR arrays (designated as CRISPR2 and CRISPR3, respectively) bracketing the cas genes. Also, the DR sequences of type I-E* are 28–29 bp in length and have consensus with four variables in the middle. Both type I-E and type I-E* share all of the eight cas genes with different relative locations of the cas6 gene, which moved from downstream of cas7-cas5 to upstream.
Interestingly, all CRISPR-Cas loci of K. pneumoniae in previous studies were identified in STs other than CC258, implicating an association between CRISPR-mediated immunity, the success of CC258 and IncF epidemic resistance plasmids. However, there are insufficient epidemiological and functional data supporting the role of CRISPR-Cas systems in swiftly spreading high-risk CC258. Here we investigate the prevalence of CRISPR-Cas systems both in 381 K. pneumoniae isolates collected from hospitals in four different provinces of China and in 207 publicly available K. pneumoniae complete genome sequences. Our results indicate an epidemic link between CRISPR-Cas systems, K. pneumoniae CC258 and IncF plasmids. Several matches for the spacers of the CRISPR array identified from plasmids suggest that these CRISPR-Cas systems are strongly associated with interfering with the survival of IncF plasmids.
Materials and methods
Media and growth of strains
LB medium was used in all experiments for the growth of bacteria and super optimal broth with catabolite repression (SOC) was used for the recovery of transformants. Bacterial strains were routinely grown at 37°C.
Clinical isolates
We randomly collected 381 non-duplicated K. pneumoniae isolates from patients at five hospitals in four provinces of China with different reported resistance rates (Figure S2) from January 2017 to February 2018. Among them, 251 were collected from Huashan Hospital (Shanghai), 27 were collected from Jinshan Hospital (Shanghai), 53 were collected from Taizhou Municipal Hospital (Zhejiang), 31 were collected from Kunming Yan’an Hospital (Yunnan) and 19 were collected from the First Affiliated Hospital of Guangxi Medical University (Guangxi). Identification and antimicrobial susceptibility testing were performed using the VITEK 2 system (bioMérieux). MLST was performed according to the protocol described on the Pasteur Institute MLST website for K. pneumoniae (https://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
PCR screening of CRISPR-Cas
The collected clinical isolates were tested for the presence of CRISPR-Cas systems by analytical PCR using the primers listed in Table S1. The presence of type I-E and type I-E* CRISPR-Cas systems was preliminarily tested by amplifying the cysH-iap and the ABC transport system-glyoxalase regions, as all of the CRISPR-Cas systems identified in the complete genome sequences of K. pneumoniae available in GenBank were located in cysH-iap or ABC transport system-glyoxalase regions, and then confirmed by amplifying the conserved genes cas1 and cas3, respectively (Figure S1).
Genome and plasmid analysis
All of the K. pneumoniae complete genome sequences publicly available (207 in total) and all of the plasmid sequences harboured in these strains (604 in total) were downloaded from GenBank in April 2018 (Dataset S1 and Dataset S2, respectively). MLST was performed by comparing with information in Institute Pasteur MLST database online (https://bigsdb.pasteur.fr/cgi-bin/bigsdb/bigsdb.pl?db=pubmlst_klebsiella_seqdef&page=sequenceQuery). Plasmid incompatibility type was determined by comparing with information in the Plasmid MLST locus/sequence definitions database (https://pubmlst.org/bigsdb?db=pubmlst_plasmid_seqdef&page=sequenceQuery). CRISPRFinder was used with default parameters to identify the CRISPR loci in the genomes and determine the number and sequences of the spacers within CRISPR arrays.11 Nucleotide BLAST was used to identify the cas gene upstream and downstream of the CRISPR loci. Nucleotide BLAST was also used to search for protospacers with a minimum of 90% identities (29/32 nt) on plasmids.
Statistics
Statistical significance was assessed by a two-tailed Student’s t-test or χ2 test using GraphPad Prism7 software (https://www.graphpad.com/). A significance level of P < 0.05 was used for all statistics.
Results
CRISPR-Cas loci are extremely rare in ST11 K. pneumoniae, but are significantly over-represented in the non-CC258 group in China
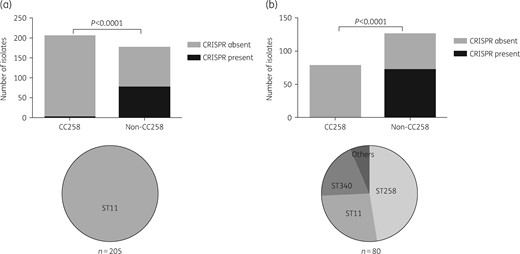
To evaluate the correlation between the absence of CRISPR-Cas systems and the dissemination of high-risk CC258 in clinical isolates, we investigated the presence of CRISPR-Cas loci in clinical isolates of K. pneumoniae. Considering the fact that the prevalence of carbapenem-resistant K. pneumoniae ranged from 0.3% to 26.9% among different provinces in China in 2017, we collected 381 K. pneumoniae isolates from hospitals in four provinces with different carbapenem resistance rates (Figure S2). Among these, the MLST STs of 53.8% (205/381) of isolates belonged to the CC258 group and all of the 205 isolates were ST11 (Figure 1a, bottom). Furthermore, CRISPR-Cas loci were extremely rare (only one positive clone among 205 tested) in ST11, being almost exclusively found among isolates of the non-CC258 group (P < 0.0001) (Figure 1a, top). Together, these results suggested an epidemic link between CRISPR-Cas systems and ST11 K. pneumoniae in China.
Presence of CRISPR-Cas loci in the CC258 and non-CC258 groups of K. pneumoniae: (a) 381 clinical isolates collected in this study and MLST of 205 clinical isolates belonging to the CC258 group; and (b) 207 completely sequenced strains available in GenBank and MLST of 80 sequenced strains belonging to the CC258 group.
CRISPR loci are frequently present in K. pneumoniae belonging to the non-CC258 group
For supporting further the finding in 381 clinical isolates of K. pneumoniae, we compiled global data of 207 complete genome sequences of K. pneumoniae. GenBank accession numbers and other information regarding the 207 isolates are provided in Dataset S1. The dataset comprises K. pneumoniae isolates identified from 18 countries (Figure S3). The countries with the largest numbers of isolates were the USA (130), China (23) and Australia (22). MLST indicated that 38.6% (80/207) of isolates belonged to the CC258 group and, within the CC258 group, ST258 was the most prevalent ST, followed by ST11 and ST340 (Figure 1b, bottom). Remarkably, CRISPR loci were absent in the K. pneumoniae strains belonging to the CC258 group, but far more common in those of the non-CC258 group (0/80 versus 71/127, P < 0.0001) (Figure 1b, top).
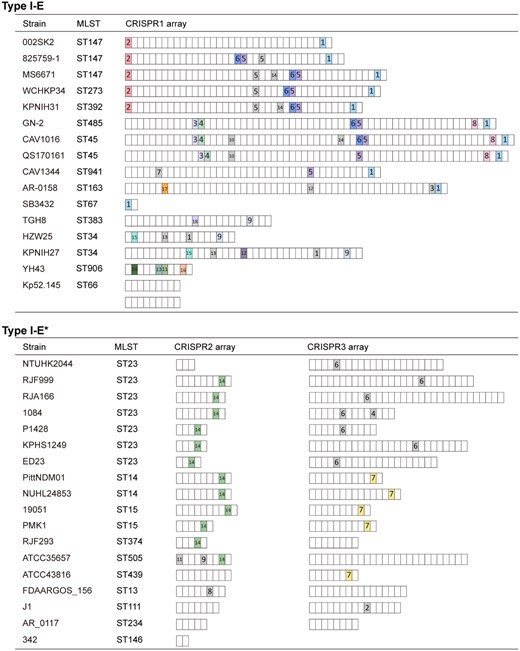
Out of the 207 completely sequenced K. pneumoniae strains available in GenBank, 34.3% (71/207) of strains contained one CRISPRFinder-confirmed CRISPR locus (Figure S1 and Dataset S1). Among the identified CRISPR loci, 56.3% (40/71) were classified as type I-E, while 43.7% (31/71) were type I-E*. Most of the identified CRISPR loci possessed both the CRISPR array and the intact cas operon, with two exceptions (K. pneumoniae YH43 and 342, which only contained the CRISPR array). These observations are similar to results of clinical isolates collected in four provinces of China, indicating a putative correlation between the CRISPR-Cas system and CC258 K. pneumoniae.
CRISPR array profiles identified in K. pneumoniae containing CRISPR-Cas loci. CRISPR1 in type I-E (top) and CRISPR2 and CRISPR3 in type I-E* (bottom). Each box represents a spacer sequence. The coloured boxes indicate the spacers matching plasmids, while the grey boxes indicate the spacers targeting the Klebsiella phages. More details about plasmid-related spacers and phage-related spacers are available in Table 1 and Table S2, respectively.
CRISPR-Cas systems target the backbone regions of IncF plasmids, the main vehicles for β-lactamase dissemination within species
The 71 identified CRISPR loci of K. pneumoniae contained 34 different layouts of CRISPR array and 415 distinct spacers (Figure 2). Out of these spacers, there were 239 distinct spacers for CRISPR1, which is greater than the total number of distinct spacers for CRISPR2 (42) and CRISPR3 (134). To determine whether these type I-E CRISPR systems were associated with dissemination of K. pneumoniae CC258, we searched for protospacers on all of the 604 plasmids (Dataset S2) existing in the 207 complete genome sequences of K. pneumoniae. Nucleotide BLAST for these spacer sequences revealed that 18 spacers matched plasmids with a minimum of 90% identities (29/32 nt) (Table 1). Ten of the 18 spacers were shared by multiple non-clonal isolates with a similar layout (Figure 2).
Spacers of the K. pneumoniae CRISRP loci matching plasmids
| Name . | CRISPR array . | Sequence (5′ to 3′) . | No. of isolates . | Protospacer location in the plasmid . |
|---|---|---|---|---|
| Spacer 1 | CRISPR1 | CAGACAGACAGCAGGCAGCAAACAGGGAAGAC | 34 | relE; non-coding region upstream of ssb and downstream of psiB and parB |
| Spacer 2 | CRISPR1 | GAGCAGGCACCCGCCGCAACGACGAAGAGCGC | 26 | ssb; non-coding region downstream of ardA (encoding an anti-restriction protein) |
| Spacer 3 | CRISPR1 | GTGGTTTGTTACCGTGTTGTGTGGCAAAAAGC | 4 | gene encoding a hypothetical protein downstream of psiA, psiB, parB and ssb |
| Spacer 4 | CRISPR1 | GAACGGAGGAATATAAGAACAAAAGCCCGCAG | 4 | gene encoding a hypothetical protein downstream of psiA, psiB, parB and ssb |
| Spacer 5 | CRISPR1 | TTAATACCAGGGGGCAGGTTCAGCAGGTCCCC | 31 | ssb; non-coding region downstream of ardA (encoding an anti-restriction protein) |
| Spacer 6 | CRISPR1 | CGATAACCGGGCGTTTCGACTGAACTCACCTC | 28 | gene encoding a hydrolase upstream of ssb |
| Spacer 7 | CRISPR3 | CCGCCGTTTAATCGCGGTGATGATATCCGGCA | 16 | gene encoding a SAM-dependent methyltransferase upstream of ssb |
| Spacer 8 | CRISPR1 | TCGTCTGAGTTCCGGCTTACGCCGTGCCGACA | 4 | gene encoding a hypothetical protein upstream of a gene encoding an anti-restriction protein |
| Spacer 9 | CRISPR1 | CCCCGTCGTCATTCGCGCATTCTGCGCACAGA | 4 | ydaB |
| Spacer 10 | CRISPR1 | TACTGCAGCAGGATGTCGTAGCCGATATAGTC | 1 | traH |
| Spacer 11 | CRISPR1 | GAAATAACCGTCTTCATTTCCACCCTCCCTCA | 1 | traN |
| Spacer 12 | CRISPR1 | TACTGAAACGGGTAATCAGCACAAATACCAAA | 1 | traT |
| Spacer 13 | CRISPR1 | GATACAGAATGGCTTCGTACAGCGACCGTTTG | 1 | traF |
| Spacer 14 | CRISPR2 | CCTGCAGCTGGCCGTCGAGCTGACGGATGCCG | 26 | gene encoding a hypothetical protein upstream of a gene encoding a DNA binding protein |
| Spacer 15 | CRISPR1 | TGCGGGCCACCAGGGTTGCAGGGTATCAATGG | 2 | finO |
| Spacer 16 | CRISPR1 | TGATTGACGCGAAGCTGCGTTATCCCAACACC | 1 | gene encoding a hypothetical protein |
| Spacer 17 | CRISPR1 | GTTTCGCACTCGCCGTTCTGACTGCTGCGCCA | 1 | gene encoding a hypothetical protein |
| Spacer 18 | CRISPR1 | TGCTTTATGGCAAATAAGAGAGGATATAACCA | 2 | gene encoding a peptidase |
| Name . | CRISPR array . | Sequence (5′ to 3′) . | No. of isolates . | Protospacer location in the plasmid . |
|---|---|---|---|---|
| Spacer 1 | CRISPR1 | CAGACAGACAGCAGGCAGCAAACAGGGAAGAC | 34 | relE; non-coding region upstream of ssb and downstream of psiB and parB |
| Spacer 2 | CRISPR1 | GAGCAGGCACCCGCCGCAACGACGAAGAGCGC | 26 | ssb; non-coding region downstream of ardA (encoding an anti-restriction protein) |
| Spacer 3 | CRISPR1 | GTGGTTTGTTACCGTGTTGTGTGGCAAAAAGC | 4 | gene encoding a hypothetical protein downstream of psiA, psiB, parB and ssb |
| Spacer 4 | CRISPR1 | GAACGGAGGAATATAAGAACAAAAGCCCGCAG | 4 | gene encoding a hypothetical protein downstream of psiA, psiB, parB and ssb |
| Spacer 5 | CRISPR1 | TTAATACCAGGGGGCAGGTTCAGCAGGTCCCC | 31 | ssb; non-coding region downstream of ardA (encoding an anti-restriction protein) |
| Spacer 6 | CRISPR1 | CGATAACCGGGCGTTTCGACTGAACTCACCTC | 28 | gene encoding a hydrolase upstream of ssb |
| Spacer 7 | CRISPR3 | CCGCCGTTTAATCGCGGTGATGATATCCGGCA | 16 | gene encoding a SAM-dependent methyltransferase upstream of ssb |
| Spacer 8 | CRISPR1 | TCGTCTGAGTTCCGGCTTACGCCGTGCCGACA | 4 | gene encoding a hypothetical protein upstream of a gene encoding an anti-restriction protein |
| Spacer 9 | CRISPR1 | CCCCGTCGTCATTCGCGCATTCTGCGCACAGA | 4 | ydaB |
| Spacer 10 | CRISPR1 | TACTGCAGCAGGATGTCGTAGCCGATATAGTC | 1 | traH |
| Spacer 11 | CRISPR1 | GAAATAACCGTCTTCATTTCCACCCTCCCTCA | 1 | traN |
| Spacer 12 | CRISPR1 | TACTGAAACGGGTAATCAGCACAAATACCAAA | 1 | traT |
| Spacer 13 | CRISPR1 | GATACAGAATGGCTTCGTACAGCGACCGTTTG | 1 | traF |
| Spacer 14 | CRISPR2 | CCTGCAGCTGGCCGTCGAGCTGACGGATGCCG | 26 | gene encoding a hypothetical protein upstream of a gene encoding a DNA binding protein |
| Spacer 15 | CRISPR1 | TGCGGGCCACCAGGGTTGCAGGGTATCAATGG | 2 | finO |
| Spacer 16 | CRISPR1 | TGATTGACGCGAAGCTGCGTTATCCCAACACC | 1 | gene encoding a hypothetical protein |
| Spacer 17 | CRISPR1 | GTTTCGCACTCGCCGTTCTGACTGCTGCGCCA | 1 | gene encoding a hypothetical protein |
| Spacer 18 | CRISPR1 | TGCTTTATGGCAAATAAGAGAGGATATAACCA | 2 | gene encoding a peptidase |
Spacers of the K. pneumoniae CRISRP loci matching plasmids
| Name . | CRISPR array . | Sequence (5′ to 3′) . | No. of isolates . | Protospacer location in the plasmid . |
|---|---|---|---|---|
| Spacer 1 | CRISPR1 | CAGACAGACAGCAGGCAGCAAACAGGGAAGAC | 34 | relE; non-coding region upstream of ssb and downstream of psiB and parB |
| Spacer 2 | CRISPR1 | GAGCAGGCACCCGCCGCAACGACGAAGAGCGC | 26 | ssb; non-coding region downstream of ardA (encoding an anti-restriction protein) |
| Spacer 3 | CRISPR1 | GTGGTTTGTTACCGTGTTGTGTGGCAAAAAGC | 4 | gene encoding a hypothetical protein downstream of psiA, psiB, parB and ssb |
| Spacer 4 | CRISPR1 | GAACGGAGGAATATAAGAACAAAAGCCCGCAG | 4 | gene encoding a hypothetical protein downstream of psiA, psiB, parB and ssb |
| Spacer 5 | CRISPR1 | TTAATACCAGGGGGCAGGTTCAGCAGGTCCCC | 31 | ssb; non-coding region downstream of ardA (encoding an anti-restriction protein) |
| Spacer 6 | CRISPR1 | CGATAACCGGGCGTTTCGACTGAACTCACCTC | 28 | gene encoding a hydrolase upstream of ssb |
| Spacer 7 | CRISPR3 | CCGCCGTTTAATCGCGGTGATGATATCCGGCA | 16 | gene encoding a SAM-dependent methyltransferase upstream of ssb |
| Spacer 8 | CRISPR1 | TCGTCTGAGTTCCGGCTTACGCCGTGCCGACA | 4 | gene encoding a hypothetical protein upstream of a gene encoding an anti-restriction protein |
| Spacer 9 | CRISPR1 | CCCCGTCGTCATTCGCGCATTCTGCGCACAGA | 4 | ydaB |
| Spacer 10 | CRISPR1 | TACTGCAGCAGGATGTCGTAGCCGATATAGTC | 1 | traH |
| Spacer 11 | CRISPR1 | GAAATAACCGTCTTCATTTCCACCCTCCCTCA | 1 | traN |
| Spacer 12 | CRISPR1 | TACTGAAACGGGTAATCAGCACAAATACCAAA | 1 | traT |
| Spacer 13 | CRISPR1 | GATACAGAATGGCTTCGTACAGCGACCGTTTG | 1 | traF |
| Spacer 14 | CRISPR2 | CCTGCAGCTGGCCGTCGAGCTGACGGATGCCG | 26 | gene encoding a hypothetical protein upstream of a gene encoding a DNA binding protein |
| Spacer 15 | CRISPR1 | TGCGGGCCACCAGGGTTGCAGGGTATCAATGG | 2 | finO |
| Spacer 16 | CRISPR1 | TGATTGACGCGAAGCTGCGTTATCCCAACACC | 1 | gene encoding a hypothetical protein |
| Spacer 17 | CRISPR1 | GTTTCGCACTCGCCGTTCTGACTGCTGCGCCA | 1 | gene encoding a hypothetical protein |
| Spacer 18 | CRISPR1 | TGCTTTATGGCAAATAAGAGAGGATATAACCA | 2 | gene encoding a peptidase |
| Name . | CRISPR array . | Sequence (5′ to 3′) . | No. of isolates . | Protospacer location in the plasmid . |
|---|---|---|---|---|
| Spacer 1 | CRISPR1 | CAGACAGACAGCAGGCAGCAAACAGGGAAGAC | 34 | relE; non-coding region upstream of ssb and downstream of psiB and parB |
| Spacer 2 | CRISPR1 | GAGCAGGCACCCGCCGCAACGACGAAGAGCGC | 26 | ssb; non-coding region downstream of ardA (encoding an anti-restriction protein) |
| Spacer 3 | CRISPR1 | GTGGTTTGTTACCGTGTTGTGTGGCAAAAAGC | 4 | gene encoding a hypothetical protein downstream of psiA, psiB, parB and ssb |
| Spacer 4 | CRISPR1 | GAACGGAGGAATATAAGAACAAAAGCCCGCAG | 4 | gene encoding a hypothetical protein downstream of psiA, psiB, parB and ssb |
| Spacer 5 | CRISPR1 | TTAATACCAGGGGGCAGGTTCAGCAGGTCCCC | 31 | ssb; non-coding region downstream of ardA (encoding an anti-restriction protein) |
| Spacer 6 | CRISPR1 | CGATAACCGGGCGTTTCGACTGAACTCACCTC | 28 | gene encoding a hydrolase upstream of ssb |
| Spacer 7 | CRISPR3 | CCGCCGTTTAATCGCGGTGATGATATCCGGCA | 16 | gene encoding a SAM-dependent methyltransferase upstream of ssb |
| Spacer 8 | CRISPR1 | TCGTCTGAGTTCCGGCTTACGCCGTGCCGACA | 4 | gene encoding a hypothetical protein upstream of a gene encoding an anti-restriction protein |
| Spacer 9 | CRISPR1 | CCCCGTCGTCATTCGCGCATTCTGCGCACAGA | 4 | ydaB |
| Spacer 10 | CRISPR1 | TACTGCAGCAGGATGTCGTAGCCGATATAGTC | 1 | traH |
| Spacer 11 | CRISPR1 | GAAATAACCGTCTTCATTTCCACCCTCCCTCA | 1 | traN |
| Spacer 12 | CRISPR1 | TACTGAAACGGGTAATCAGCACAAATACCAAA | 1 | traT |
| Spacer 13 | CRISPR1 | GATACAGAATGGCTTCGTACAGCGACCGTTTG | 1 | traF |
| Spacer 14 | CRISPR2 | CCTGCAGCTGGCCGTCGAGCTGACGGATGCCG | 26 | gene encoding a hypothetical protein upstream of a gene encoding a DNA binding protein |
| Spacer 15 | CRISPR1 | TGCGGGCCACCAGGGTTGCAGGGTATCAATGG | 2 | finO |
| Spacer 16 | CRISPR1 | TGATTGACGCGAAGCTGCGTTATCCCAACACC | 1 | gene encoding a hypothetical protein |
| Spacer 17 | CRISPR1 | GTTTCGCACTCGCCGTTCTGACTGCTGCGCCA | 1 | gene encoding a hypothetical protein |
| Spacer 18 | CRISPR1 | TGCTTTATGGCAAATAAGAGAGGATATAACCA | 2 | gene encoding a peptidase |
Out of the 604 plasmids in the 207 complete genomes of K. pneumoniae, 218 plasmids were identified as being matched with one spacer at least, while 386 plasmids were protospacer negative. Analysis of plasmid incompatibility type indicated that the vast majority of protospacer-positive plasmids belonged to the IncF group with divergent replicon types (e.g. IncFIIK, IncFII, IncFIIY, IncFIA, IncFIB and IncFIIS), but this group was less common in protospacer-negative plasmids [174/218 (79.82%) versus 51/386 (13.21%), P < 0.0001; Figure S4]. Also, the protospacer-positive plasmids were far more common in the IncF group compared with the non-IncF group (P < 0.0001) (Figure S5). These protospacer-positive IncF plasmids were named CRISPRT-IncF plasmids (CRISPR-targeted IncF plasmids). Most of the 18 spacers matched about 35%–65% of plasmids in the CRISPRT-IncF group, with the exception of spacers 4 and 8, showing a decreased proportion, and spacers 15 to 18 matching no more than two plasmids (Figure S5 and Dataset S2). Spacers 1, 2 and 3 accounting for a high proportion often corresponded to multiple positions in a single plasmid (Dataset S2). Also, each individual CRISPRT-IncF plasmid could be targeted by multiple spacers and the number of distinct spacers ranged from 1 to 13 (11 mostly), such as the blaKPC-bearing plasmid pKpQIL-531 in ST258 and pKPHS2 in ST11 (GenBank accession no. CP009875 and CP003224, respectively; Figure S6 and Dataset S2). These data demonstrate that CRISPR-Cas systems and CRISPRT-IncF plasmids presumably play a role in forming the high-risk CC258.
Next, we explored the region extending from 20 bp upstream to 20 bp downstream of each spacer match on the plasmids. All of the 18 spacers matched the backbone regions shared by most IncF plasmids (Table 1). The spacers 1 to 9 and spacer 14 corresponded to a plasmid stability region, either ssb or the region adjacent to ssb, parB, ardA, psiA and psiB. The spacers 10 to 13 matched genes involved in plasmid propagation, including traH, traN, traT and traF.
Together, these data show that the CRISPR-Cas systems of K. pneumoniae target IncF plasmids, mostly in backbone regions associated with plasmid stability and propagation.
Discussion
Several global epidemiological investigations have shown that the international high-risk clone of K. pneumoniae CC258 and the associated IncF plasmid contribute significantly to pandemics of K. pneumoniae, especially KPC-producing K. pneumoniae.2,12,13 During the past decade, many studies have attempted to figure out factors leading to the epidemiological advantages of the CC258–IncF linkage over other STs and/or CC258 carrying non-IncF plasmids.12,13 CRISPR-Cas systems, providing adaptive immunity against invading MGEs, play an important role in the interactions between plasmids and hosts. Type I-E CRISPR-Cas systems of K. pneumoniae, contained in the genomes of non-CC258 isolates and containing spacers targeting IncF plasmids, are an important factor connecting the host (CC258) and the plasmid (IncF). Our findings suggest a role for type I-E CRISPR-Cas systems in the dissemination of the CC258–IncF linkage. As CRISPR-Cas systems presumably interfere with the uptake and survival of IncF plasmids within non-CC258 isolates, these plasmids are more prone to transfer to CC258 isolates without a CRISPR-Cas system. We did find 63 protospacer-positive plasmids of special interest, which existed in CRISPR-positive isolates of the non-CC258 group (Dataset S2). Further analysis indicated that the majority of these protospacer-positive plasmids (36/63) could not be targeted by native CRISPR-Cas systems. The other 27 cases might be involved with CRISPR tolerance, in which the CRISPR targeting is transiently non-lethal.14
Spacers are key elements of CRISPR-mediated immunity, as they memorize an organism’s encounters with specific MGEs, acquired as a result of a previous unsuccessful infection.6,7,15 This memory enables the recognition and neutralization of the invaders upon subsequent infections.7 The 18 spacers corresponding to CRISPRT-IncF plasmids target either the region adjacent to ssb, parB, ardA, psiA and psiB, or tra genes (Table 1). The ssb, parB and ardA genes, encoding the single-stranded binding protein, partitioning protein and anti-restriction protein, respectively, encourage the maintenance and stability of the plasmid.16 The tra genes are indispensable for IncF plasmid conjugation and psiA and psiB help plasmid transfer by inhibiting the host SOS response during conjugation.16 These genes involving plasmid stability and propagation are often shared by the CRISPRT-IncF group and are advantageous to plasmid transfer and survival.16 Notably, the majority of the 18 spacers were identified in CRISPR1 of type I-E and the number of distinct spacers for CRISPR1 is greater than the figure for CRISPR2 and CRISPR3 (Table 1 and Figure 2), reflecting the relatively more important role for type I-E in the dissemination of IncF plasmids. In comparison with 18 spacers corresponding to more than 75% of IncF plasmids, 14 other spacers were identified as targeting very rare Klebsiella phages (6.3%, 9/143) (Figure 2 and Table S2), reflecting an important role for CRISPR-Cas in interfering with the uptake and survival of CRISPRT-IncF plasmids.
Although the function of type I-E systems has never been questioned due to the finding that type I-E cas genes are repressed by the global regulator H-NS under laboratory conditions,17 this transcription repression was exerted only in stationary-phase cells.18 Moreover, H-NS-mediated repression of CRISPR-based immunity can be relieved and/or counteracted by a variety of antagonistic factors (e.g. Ler, LeuO and RovA).17,19 Indeed, a study by Lin et al.9 demonstrated that K. pneumoniae CRISPR-Cas systems led to a significant reduction in plasmid transformation.
In conclusion, our findings suggest that the absence of a CRISPR-Cas system in CC258 is closely associated with the prevalence of IncF plasmids in this CC. To the best of our knowledge, such an association has not been investigated or proposed previously for the global success of the K. pneumoniae linkage CC258–IncF. In this study, clinical isolates collected in China were investigated. A large-scale study including more countries and regions on the relationship between CC258, IncF plasmids and CRISPR-Cas systems is expected to provide more evidence. Also, the prevalence of the IncF plasmid and related spacers/protospacers would need to be investigated in clinical isolates. Importantly, our findings suggest a role for type I-E CRISPR-Cas systems in the dissemination of the high-risk linkage K. pneumoniae CC258–IncF.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (grant numbers 81871692, 81572031 and 31670074).
Transparency declarations
None to declare.
References
Author notes
Yu Tang, Pan Fu, Ying Zhou and Yingzhou Xie contributed equally to this work.