-
PDF
- Split View
-
Views
-
Cite
Cite
I. Cervelló, J.A. Martínez-Conejero, J.A. Horcajadas, A. Pellicer, C. Simón, Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers, Human Reproduction, Volume 22, Issue 1, 1 January 2007, Pages 45–51, https://doi.org/10.1093/humrep/del332
Close - Share Icon Share
Abstract
BACKGROUND: The endometrium, lining of the uterus, is a highly active organ that is remodelled periodically during the lifespan. Different studies suggest the presence of an adult or progenitor stem cell (PSC) population in this tissue because of its cyclic regenerative capacity. METHODS: In this study, we aim at identifying and localizing the putative PSC population in the murine uterus using the 5-bromo-2′-deoxyuridine (BrdU) labelling method to detect label-retaining cells (LRCs) that cycle slowly. Uteri from BrdU-treated mice were analysed via single and double immunohistochemistry to co-localize them with the markers of undifferentiation already described such as c-KIT and POU5F1 (also known as OCT-4). Finally, we confirmed the presence of the indicated markers at mRNA level. RESULTS: We observed the presence and gradual decrease of LRCs in the endometrium during the lifespan of the mice. In adulthood, the LRC population decreased notably and remained in the lower region of the stroma in the murine endometrium. Some of the endometrial LRCs co-localized with c-KIT and POU5F1. PCR and nested-PCR confirmed the presence of these undifferentiated markers. CONCLUSIONS: We demonstrated that the murine endometrium possesses LRCs with the features of a putative PSC population localized at the lower region of the stroma.
Introduction
An adult or progenitor stem cell (PSC) population is a relatively undifferentiated cluster of cells present in a specific tissue/organ that renews itself and differentiates into specialized cell types (Fuchs et al., 2004). These cells are usually in the G0 phase and are located in a protective environment named the niche. PSCs proliferate through asymmetric cell division, giving rise to one replacement stem cell and one transit amplifying cell (Watt and Hogan, 2000). The interactions with the stem cell niche are crucial to this process (Zhang et al., 2003).
PSCs have been isolated from several tissue sources, including the central nervous system (Reynolds and Weiss, 1992; Gage, 2000), bone marrow (Prockop, 1997; Pittenger et al., 1999; Weissman, 2000), retina (Tropepe et al., 2000) and skeletal muscle (Gussoni et al., 1999; Jackson et al., 1999), suggesting that most adult tissues potentially contain PSC population. This specific cell cluster presents a diverse differentiation repertoire (Joshi and Enver, 2002) ranging from unipotential adult stem cells capable of generating one specific cell type (Alison et al., 2004) to oligopotent cells that produce a limited range of differentiated cell lineages according to their location (Beltrami et al., 2003; Sanai et al., 2004). Nevertheless, compelling evidence exists for the pluripotency of several PSCs, including multipotent adult progenitor cells (MAPCs) from bone marrow (Jiang et al., 2002).
Human and mouse endometrium undergoes cyclic changes of growth, differentiation and regression during every reproductive cycle. Two research groups have suggested the presence of PSCs in the human endometrium (Chan et al., 2004; Cho et al., 2004; Schwab et al., 2005). This assumption is based on the identification of stem cell markers (c-KIT/CD117 and CD34) in the endometrial cells (Cho et al., 2004) and the presence of rare clonogenic epithelial and stromal cells with high proliferative potential (Chan et al., 2004; Schwab et al., 2005). The existence of label-retaining cells (LRCs) in murine endometrium has been recently described (Chan and Gargett, 2006).
The present study aims not only at identifying the LRC population in the mouse endometrium but also at co-localizing it with undifferentiation markers in a new attempt to characterize this special subset of cells. The localization of this cell population was carried out using the LRC method, consisting of an injection of 5-bromo-2′-deoxyuridine (BrdU), combined with co-localization using typical markers of undifferentiation: c‐KIT (Majumder et al., 1988; Ivanova et al., 2002) and Oct-4 (Hansis et al., 2001).
We have demonstrated that these murine endometrial LRCs have the features of PSCs with the expression of typical undifferentiated marker.
Materials and methods
LRC method with BrdU
Three-day-old mice (strain ICR [CD-1], Harland, Barcelona, Spain) were injected intraperitoneally with BrdU (Sigma-Aldrich, Spain) (50 µg/g of corporal weight) for 3 consecutive days (twice a day, at 08:00 and 17:00 hours), following established protocols (Taylor et al., 2000). A control group (n = 13) was killed 24 h before the last injection of BrdU to test the incorporation of the molecule. The study group (n = 48) was killed on day 21 (weaning), 35 (sexual maturity), 49 (adulthood) and thereafter on days 64, 77, 83, 90, 98, 106 and 112 to localize LRCs throughout the different stages of adulthood. This experiment was repeated twice, and a total of at least four animals were killed at each time point. Cells retaining the label after 8–10 weeks (adulthood) were identified as LRCs. The uterus and the liver were removed from BrdU-treated mice (Wang et al., 2003) (positive control) and untreated animals (negative control) for LRC identification, immunohistochemistry and RNA isolation analysis.
All studies were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. Protocols for the handling of animals and experimental procedure were approved by the Ethical Animal Committee of the School of Medicine of Valencia University.
Detection of slow-cycling cells
Paraformaldehyde-fixed and paraffin-embedded organs were sectioned and mounted on glass slides coated with Vectabond™ (Vector Laboratories, Burlingame, CA, USA). After deparaffination and rehydration, sections were rinsed in citrate buffer (pH 6, 95°C) for antigen retrieval. In the case of BrdU-labelled cells, it is necessary to perform DNA denaturation using formamide 60% in 2× SSC at 54°C and HCl 2N in phosphate-buffered saline (PBS) at 37°C, as anti-BrdU identifies BrdU (but not thymidine) in single-stranded DNA (Hayashi et al., 1988). Non-specific binding was avoided by blocking with non-fat milk (50 mg/ml in TBS). Sections were then incubated overnight at 4°C with anti-BrdU 5 µg/ml (FITC-conjugated, Becton Dickinson) and counterstained with 6-diamidino-2-phenylindole (DAPI) or incubated with anti-BrdU (DakoCytomation) and processed by the avidin–biotin–peroxidase complex method (Dako) following manufacturer’s instruction and counterstained with haematoxylin. Immunolocalization of the LRCs during the mouse lifespan (days 7–112) was visualized and photographed using an Olympus 35 mm camera attached to a fluorescence microscope (Olympus Provis AX70, Olympus, Optical España S.A.).
Immunoco-localization with undifferentiated markers
Tissue sections from the uterus, the liver and human embryonic stem cell (hESC) line VAL-1 (Simon et al., 2005) (positive controls) were analysed using immunohistochemistry to co-localize BrdU-retaining cells with the markers of undifferentiation such as POU5F1 (also known as OCT-4) and c-KIT. Slides were co-incubated overnight at 4°C with anti-BrdU 5 µg/ml, POU5F1 0.2 µg/ml (Chemicon International, monoclonal antibody) and c-KIT 0.2 µg/ml (Santa Cruz Biotechnology, goat polyclonal antibody). They were incubated for 30 min with TRITC-conjugated secondary antibodies (Sigma-Aldrich). Immunoco-localization of the specific cell populations throughout the mouse lifespan was visualized and photographed using an Olympus 35 mm camera attached to a fluorescence microscope.
RNA isolation
RNA extraction was performed according to the method described by Chomczynski and Sacchi (1987), with minor modifications using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). Briefly, each tissue was weighed, and 500 µl of Trizol reagent was added to every 25–50 mg. Total RNA was separated from DNA and proteins by adding chloroform and was precipitated with isopropanol (overnight, –20°C). The precipitate was washed twice in ethanol, air-dried and resuspended in 75% diethylpyrocarbonate (DEPC)-treated water. RNA was quantified by spectrophotometry on a SmartSpec 3000 spectrophotometer (Biorad, Barcelona, Spain). A260/A280 ratios for all samples used varied between 1.6 and 1.9.
Reverse transcription
Reverse transcription (RT) was carried out using the Advantage RT-for-PCR kit (Clontech, Palo Alto, CA, USA). The mastermix per sample was prepared as follows: 5× reaction buffer, dNTP mix (10 mM each), recombinant RNase inhibitor and Moloney-murine leukaemia virus (MMLV) reverse transcriptase. In short, 1 µg of each sample was diluted in DEPC-treated water with oligo (dT). The mixture was then heated for 2 min at 70°C and kept on ice until the mastermix was added. For each RT, a blank was prepared using all the reagents except the RNA sample, for which an equivalent volume of DEPC-treated water was substituted. The RT blank was used to prepare the PCR blank (below). Once all the components were mixed, the samples were incubated for 1 h at 42°C and heated for 5 min at 94°C to prevent cDNA synthesis and destroy DNase activity. The product was diluted with DEPC-treated water to a final volume of 100 µl and stored at –20°C until PCR and nested-PCR analyses were performed.
PCR and nested-PCR analysis
Pou5f1 primers for nested-PCR (fw-out 5′-AGGAAGCCGACAACAATGAGA-3′, rev-out 5′-CCCTCAGGAAAAGGGACTGA-3′, fw-in 5′-GGAAGAGAAAGCGAAC TAGCA-3′ and rev-in 5′-GGACTGAGTAGAGTGTGGTGA-3′) were designed according to database and software. PCR primers for c-Kit (forward 5′-CTGGTGGTTCAGAGTTCCATAGAC-3′ and reverse 5′-TCAACGACCTTCCCGAAGGCACCA-3′) were obtained as described by Lasalle et al. (1999) to amplify murine and human c-Kit. β-Actin expression was used as RNA as a housekeeping gene for normalization and forward 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′ and reverse 5′-CGGTGAGGATCTTCATGAGGTA-3′ were used as primers. PCR and nested-PCR were performed using a BIOMETRA thermal cycler.
PCR was carried out with 4 µl of RT product to which 21 µl of PCR mix [1.25 µl of 25 mM MgCl2 solution, 2.5 µl of 10× PCR buffer, 15.5 µl of DEPC-treated H2O, 0.5 µl of 10 mM dNTP, 0.5 µl of 3′ + 5′ primer mix of 20 µM each and 0.25 µl of Taq DNA polymerase (Biotaq, Bioline, London, UK)] was added. PCR was performed at 61°C: 40 cycles for c-Kit and β-Actin.
For the first round of nested-PCR, 4 µl of RT was added to the PCR mix. PCR for Pou5f1 was performed at 62°C, in 30 cycles. For the second round of PCR, 5 µl of the first-round PCR was added to the PCR mix. PCR was carried out at 62°C, in 20 cycles.
H2O substituted cDNA as the negative control for each reaction, whereas mouse liver cDNA was used as the positive control for c-Kit and murine testis was used as the positive control for Pou5f1. PCR and nested-PCR products were analysed by means of gel electrophoresis in 2% (w/v) agarose gel containing 0.5 µg/ml of ethidium bromide. The bands were isolated and sequenced to verify the identity of the PCR products.
Cell counts
Four-micrometre sections were stained with the indicated antibodies and counterstained with Mayer’s haematoxylin or DAPI, respectively, to visualize all the cells present in this section to perform the enumeration of the positive cells. Cells were counted by using the ImageJ software. At least, 2000 cells were counted for each time point against DAPI- and haematoxylin-stained nuclei. Labelling index and percentages were determined by the coefficient between the positive cells for the specific antibodies and the total number of cells in each slide determined by haematoxylin nuclear staining or DAPI (fluorescent technique). Data are expressed as mean and standard errors.
Results
Localization of BrdU-retaining cells in the murine endometrium
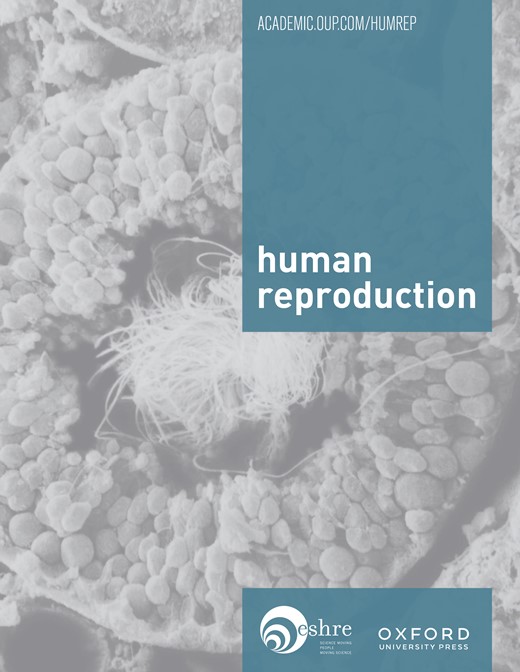
BrdU is a thymidine analogue that is incorporated into the genomic DNA during the replication phase of mitotic cycle labelling the new cells. Thereafter, the BrdU signal progressively decreases in each division. The retaining of the labelling in some populations indicates either no division or very low rate of division, one of the most important characteristics of the adult/progenitor stem cells. BrdU+ cells were identified by nuclear-localized green fluorescence. At day 7, all epithelial and stromal cells stained positive for BrdU (Figure 1). LRCs decreased sharply in the endometrial tissue of prepubescent animals (days 21–49). By day 21, the epithelial compartment had completely lost the BrdU signal and the number of BrdU-retaining cells in the stroma had diminished greatly (Figure 1). In the adulthood, from day 49 onwards (54–64–68–77–84–90–98–106–112 and 118), only a few BrdU-retained cells were present in the stromal compartment and localized at the lower region (Figure 1). Liver tissue was used as a positive control for BrdU incorporation, because adult stem cells have been already described in liver murine oval cells (Wang et al., 2003), and there was no signal in endometrial tissue sections from untreated animals (negative controls) (Figure 1).
5-Bromo-2′-deoxyuridine (BrdU)-retaining cells in the mouse endometrium. (A) At day 7, post-injection, BrdU-retaining cells are present in both epithelial and stromal compartments. (B) In 21-day-old mouse, BrdU was selectively retained (green nuclear staining) in cells at the stromal compartment. (C) In 49-day-old mouse after a chase of 7 weeks and (D) in 112-day-old mouse followed by a chase period of 15 weeks, green labelled cells were restricted to the lower region of the stroma. (E) Liver was used as positive control, and (F) negative control was mouse uterus without BrdU treatment. (G) Life table of the percentage of BrdU-retaining cells in the murine endometrium throughout the mouse lifespan. GE, glandular epithelium; LE, luminal epithelium; PA, hepatic portal area; S, stroma. All panels are ×400 magnification.
The dynamics of the presence of BrdU-retaining cells in the murine endometrium throughout the lifespan can be assessed in Figure 1G. There was a sharp decrease of LRCs from 34 ± 9.6 to 8.6 ± 5.3% between prepubescence and adulthood; the epithelium lost completely the label around week 3; LRCs slowed down from 8.6 ± 5.3 to 1.7 ± 0.8% between days 49 and 112.
Co-localization of LRCs with undifferentiation marker c-Kit at the protein and m-RNA levels
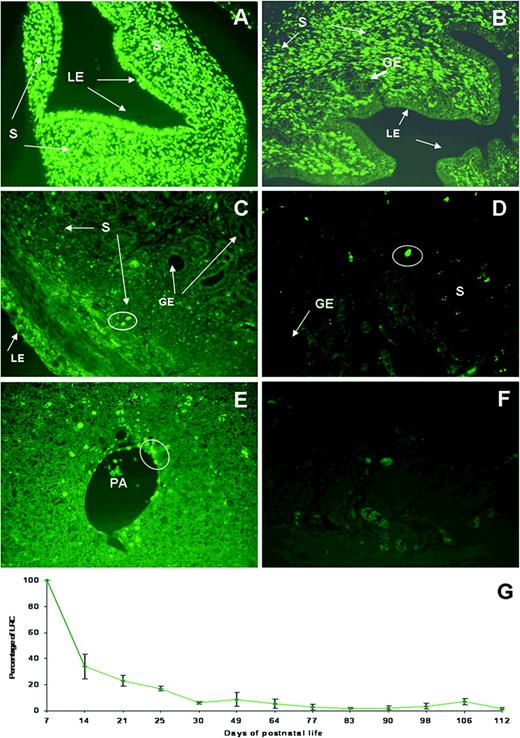
The stem cell factor receptor c-Kit was described in haematopoietic stem cell (HSC) population in adult and expressed in pluripotent cells (Jackson et al., 2001). c-KIT+ cells were identified through cytoplasm-localized red fluorescence. The co-localization of BrdU-retaining cells with c-KIT/CD117 demonstrated that this undifferentiation marker was present in some of the stromal LRCs located in the lower region of the murine endometrium (Figure 2). Liver tissue was used as a positive control for the c-KIT signal, and primary antibody was omitted in murine endometrium as negative control (Figure 2). The percentage was expressed as mean ± standard error of BrdU+/c-KIT+ cells compared with the total BrdU+ cell population and is presented in Figure 2 There was a continuous decrease of the co-localized BrdU+/c-KIT+ population throughout the lifespan, up to 0.3 ± 0.4% of the total BrdU population on the last days analysed (day 112).
Co-localization of 5-bromo-2′-deoxyuridine (BrdU) cells with the undifferentiation marker c-KIT in the murine endometrium. (A) Green BrdU-labelled cells in the stromal endometrium at day 83. (B) c-Kit+ cells in the same section as (A) (red cytoplasmic staining). (C) Co-localized cells with both green BrdU and red because of c-Kit, in the stromal endometrium. (D) Positive control was oval cells of the portal area of the murine liver, and (E) murine endometrium with the deletion of the first antibody was used as negative control. (F) A table with the percentage of BrdU-retaining cells and BrdU-retaining cells co-localized with c-KIT during mouse lifespan. GE, glandular epithelium; LE, luminal epithelium; PA, hepatic portal area; S, stroma. Magnification ×400.
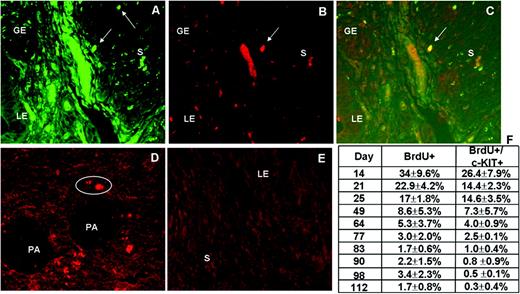
To further prove the presence of c-Kit mRNA, the mouse uterus was analysed during the prepubescent period (day 21) and adulthood (day 50) via RT–PCR, using the primers and conditions indicated above. c-Kit mRNA expression was detected in the uterus during the analysed life stages. Liver tested positive, whereas no product was detected in human skeletal muscle or distilled water (Figure 3A). The identity of the PCR product was verified by sequence analysis.
PCR and nested-PCR analysis for c-Kit and Pou5f1 in the murine uterus. (A) Upper panel: PCR shows a 389-bp band corresponding to c-Kit product. Lower panel: the expression of the housekeeping gene β-Actin is shown. (B) Upper panel: nested-PCR for Pou5f1 in the murine uterus. A band corresponding to 318 bp appears when a second round of PCR was performed using the product of the first amplification. Lower panel: housekeeping expression is shown. All bands obtained by PCR and nested-PCR were confirmed by sequentiation analysis. Lanes: Ud21, murine uterus at day 21; Ud50, murine uterus at day 50. Liver corresponds to murine liver as positive control; Musc, human skeletal muscle as negative control; tes, murine testis as positive control; FSK, human foreskin as negative control.
Co-localization of LRCs with undifferentiation marker POU5F1 at the protein and mRNA levels
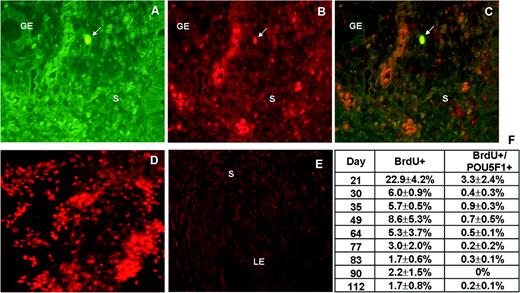
POU5F1 is a nuclear transcription factor of undifferentiation. POU5F1+ cells were identified through nuclear red fluorescence staining and co-localized with BrdU-retained cells (Figure 4). Human ESC line VAL-1 was used as a positive control and murine endometrium with the deletion of the first antibody as negative control (Figure 4). This undifferentiation marker was localized in the lower region of the stromal compartment in different stages analysed, but at lower percentage compared with c-KIT. At day 112, it remains a small subset of 0.2 ± 0.1% POU5F1+ cells of the BrdU population (BrdU+/POU5F1+) (Figure 4).
Co-localization of 5-bromo-2′-deoxyuridine (BrdU) cells with the undifferentiation marker POU5F1 in the mouse endometrium. (A) Arrow indicates green BrdU-labelled cells in the stromal endometrium at day 49. (B) POU5F1+ cells (red staining) can be visualized in the same section as (A). (C) Arrow indicates the double-labelled cells (BrdU+/POU5F1+) in the murine endometrium. (D) A human embryonic stem cell line (VAL-1) was used as positive control, and (E) mouse endometrium with the deletion of the first antibody was used as negative control. (F) The percentage of BrdU-retaining cells, and BrdU-retaining cells co-localized with POU5F1 during mouse life. GE, glandular epithelium; LE, luminal epithelium; PA, hepatic portal area; S, stroma. All panels are ×400 magnification.
Nested-PCR revealed the presence of Pou5f1 mRNA in the mouse uterus during the prepuberal period (day 21) and adulthood (day 50) (Figure 3B). PCR products were verified by sequence analysis.
Discussion
Adult/progenitor stem cells constitute a rare population of resident cells found in many tissues. They are located in specific niches and are crucial for the maintenance of tissue homeostasis (Fuchs and Segre, 2000). A reliable method for identifying the endometrial PSCs is based on the fact that these cells are slow-cycling and, therefore, can be considered as LRCs (Bickenbach, 1981). In this procedure, a tissue is long-term labelled with 3H-thymidine or BrdU and followed by a ‘chase’ period in which the label is diluted out from rapidly dividing (transit amplifying) cells and retained by the slow-cycling, which are identified as LRCs. This approach is validated by the absence of LRCs in tissues customarily regarded as self-sufficient, such as the corneal epithelium, and the detection of these cells exclusively in the limbus of the peripheral cornea (Cotsarelis et al., 1989). Furthermore, the labelling system has been improved using BrdU instead of 3H-TdR. BrdU is a thymidine analogue that is incorporated into the genomic DNA during the replication phase of the mitotic cycle labelling the new cells (Braun et al., 2003). It can be detected as an intense, green nuclear stain, which in turn permits the identification of LRCs and cells derived from LRCs after several rounds of division (Taylor et al., 2000).
In these experiments, we identified an LRC population in the mouse endometrium located in the lower region of the stromal compartment. Moreover, these cells exhibited undifferentiation markers. We found that the percentage of the LRCs in the mouse endometrium during adulthood slowly decreased from 8.6 ± 5.3% at day 49 to 1.7 ± 0.8% at day 112 and was restricted to the stromal compartment. The epithelium lost the BrdU labelling around 3 weeks of life (Figure 1). Gargett et al. using miniosmotic pumps containing 3.5 mg/ml of BrdU in 0.9 saline described that LRCs are present in the epithelial compartment up to 4 weeks (0.2 ± 0.2), but no definitive number is reported. Using the same method as in our study, the percentage of epithelial LRCs declined progressively from ∼72 to 2% over the course of the first 4 weeks (Chan and Gargett, 2006), then reaching 0% at 12-week chase. In the present work, epithelial LRCs were also detected up to 3 weeks. Nevertheless, because 3 or 4 weeks are just the initiation of puberty, the presence and putative function of the epithelial LRC population in the adulthood remains unclear. This percentage is higher than noted in many organs such as the bone marrow (De Rooij and Van Bragt, 2004), the prostate (Tsujimura et al., 2002), the lung (Summer et al., 2003) and the pancreas (Duvillie et al., 2003), and similar to that in the skin in mice (1.1%) (Montanaro et al., 2003, 2004) and humans (0.01–5.4%). A possible explanation for the significant presence of PSCs in the endometrium is because of its being a highly active tissue. In most mammals, the lining of the uterine cavity, the endometrium, builds periodically a new layer which, if no pregnancy occurs, is shed or reabsorbed. In the mouse, remodelling and reorganization in this tissue occurs at each estrous cycle. PSCs present in the uterus may be involved in the changes implicated in endometrial development. Our findings are consistent with the recent publication describing endometrial LRCs in mouse endometrium (Chan and Gargett, 2006).
Another remarkable feature is the fact that the percentage of endometrial PSCs decreases with ageing (Figure 1). This phenomenon has also been demonstrated in the skin (Yano et al., 2005) and indicates an extenuation of this population during the lifespan, suggesting that asymmetric division is compromised in endometrial PSCs.
The concept of specialized niches was developed within the haematopoiesis field, where a great deal is known about the in vitro conditions that mimic an in vivo environment. Secreted molecules, such as colony-stimulating factor and stem cell factor [Kit ligand (KitL)], play important roles in cell survival (Lotem and Sachs, 1993; Smith et al., 2001). Recent research has highlighted this critical concept by demonstrating that matrix metalloproteinase-9 (MMP-9) expression is induced in bone marrow cells that release soluble KitL, permitting the transfer of endothelial and haematopoietic stem cells from the quiescent to the proliferative niche. Bone marrow ablation induces stromal cell-derived factor-1, which up-regulates MMP-9 expression and causes shedding of soluble KitL and recruitment of c-KIT+ stem/progenitors (Shinde Patil et al., 2005).
The proto-oncogene c-Kit/CD117 encodes a 145-kDa transmembrane tyrosine kinase receptor (CD117), and this ligand is a stem cell factor (Lev et al., 1994). Kit ligand would appear to exert a wide range of effects on germ cells, melanocytes, mast cells and primitive haematopoietic cells (Williams et al., 1992). c-KIT has also been described as an undifferentiation marker in the human endometrium (Cho et al., 2004). Although not detected in the human fetal endometrium, it has been expressed with moderate staining in the stroma and basalis glands of the endometrium throughout the lifetime in humans (Cho et al., 2004). We co-localized the LRC population present in the murine endometrium with c-KIT. The expression of c-KIT was restricted to cells in the lower region of the stroma and represents 0.32% of the LRCs in the adulthood (day 112).
POU5F1 has been identified as the nuclear transcription factor expressed in embryonic stem cells, germ stem cells and in the germ cell line in mice (Scholer, 1991). Pou5f1 encodes a transcription factor involved in the maintenance of undifferentiation and is widely regarded as a hallmark of pluripotent stem cells (Goodell et al., 1996). Upon differentiation to somatic lineages, the expression of Pou5f1 subsides rapidly. In this study, we have co-localized POU5F1 cells in some of the endometrial LRC population, pointing towards the highly undifferentiated status of some cells in the PSC population. The expression of POU5F1 was restricted to cells in the lower region of the murine stroma and represented only 0.19% of the LRCs at day 112.
In summary, we demonstrated the existence of LRCs with the features of a PSC population in the murine endometrium. This undifferentiated subset of cells has been characterized by the LRC method and co-localized with typical stem cell markers c-KIT and POU5F1.
Acknowledgements
We thank Inma Noguera for expert advice at the Animal Central Facilities Core and Ana Cervero and Eva Sánchez from Fundación IVI for valuable advice. This work has been financed by grant SAF 2004–00204, allocated by the Ministerio de Educación y Ciencia of the Spanish government (PI C.S.). I.C. is the recipient of a predoctoral grant from the IVI Foundation and J.A.M.-C. is the recipient of FPU Grant number AP2002–1028 from the Ministerio de Educación y Cultura. This work has been partially presented at the 52nd (2005) and 53rd (2006) Annual Meeting of the Society of Gynaecologic Investigation (SGI) in Los Angeles (USA) and Toronto (Canada).