-
PDF
- Split View
-
Views
-
Cite
Cite
J. Kline, A. Kinney, A. Kelly, M.L. Reuss, B. Levin, Predictors of antral follicle count during the reproductive years, Human Reproduction, Volume 20, Issue 8, August 2005, Pages 2179–2189, https://doi.org/10.1093/humrep/dei048
Close - Share Icon Share
Abstract
BACKGROUND: We sought to identify indicators of antral follicle count which would be serviceable to clinicians seeking to estimate the number of ovarian follicles without relying on sonographic counts. METHODS: We examined the relations of chronological age and four potential indicators of ovarian age—ovarian volume, FSH, dimeric inhibin B and estradiol—to antral follicle count in 176 recently pregnant women. We identified the regression models which best predict low antral follicle count (≤10 follicles). RESULTS: Chronological age, ovarian volume, FSH and inhibin B were each significantly associated with antral follicle count. Fifty-three (30.1%) women had ≤10 antral follicles. In the total sample, at the cutpoint corresponding to 80% sensitivity, the positive predictive value for a regression model with all four variables was 60%. All regression models performed less well in women <35 years (13.9% with low count) than in women ≥35 years (52.0% with low count). In older women, the positive predictive value for the model with all four variables was 79%, compared with 60% for a model with chronological age alone. CONCLUSIONS: Our models provide a basis for advising women aged ≥35 years who are either trying to conceive or wish to learn whether they may postpone childbearing.
Introduction
Many women defer childbearing to the later years, raising their risk of adverse pregnancy outcomes associated with ovarian ageing. In particular, rates of fecundity (the physiological capacity to have a live birth) and fertility (the number of actual live births) begin to decline rapidly when women reach the mid–late 30s (Howe et al., 1985; Menken et al., 1986). While social and medical factors (e.g. contraception, voluntary sterility, coital frequency) influence these rates, some of the decline is biologically determined. Data suggest that follicular and ovarian, rather than uterine, factors are the primary determinants of decreasing fecundability (the physiological capacity to conceive) during a woman's 30s and 40s. For example, although implantation and clinical pregnancy rates for women receiving donor ova do not decline until about age 50 years (CDC, 2000; Toner et al., 2002), pregnancy rates for women attempting assisted reproduction with their own ova start to decline when they are in their mid-30s (CDC, 2000).
Studies of autopsy and surgical specimens show that the germ cell population, which is largest during fetal development (∼7×106), decreases approximately exponentially with chronological age (Block, 1952; Baker, 1963; Thomford et al., 1987; Faddy et al., 1992; Leidy et al., 1998; Westhoff et al., 2000). A similar decline is evident for antral follicles, the small fraction (<0.5%) of the total pool that develops and enlarges during each menstrual cycle. Sonographic studies confirm that antral follicle count declines with chronological age in women of reproductive age (Reuss et al., 1996; Scheffer et al., 1999; Broekmans et al., 2004; Kline et al., 2004). Moreover, a detectable decline in follicle count may precede detectable changes in hormone levels (Scheffer et al., 1999; Kline et al., 2004). It is reasonable to infer that the decline in fecundity with chronological age reflects, at least in part, the diminishing supply of oocytes. Among infertile women, indicators of the size of the oocyte pool supplement chronological age as predictors of the response to assisted reproductive technologies. While findings are not entirely consistent (Bukman and Heineman, 2001; Bancsi et al., 2003), antral follicle count, ovarian volume and pretreatment levels of FSH and inhibin B have each been associated with ovarian response to hormonal stimulation and clinical pregnancy rates.
In this paper we assume that antral follicle count is the best indicator of the size of the underlying follicle pool. Obtaining high quality data is, however, labour intense. While follicles can be counted with moderate to high reliability in research settings (Scheffer et al., 2002; Kline et al., 2004), similar results are less likely in clinical practice (Hansen et al., 2003), at least until specialized (potentially automated) three-dimensional applications are developed and widely available. We therefore sought to identify indicators other than antral follicle count which would be serviceable to clinicians seeking to estimate the size of the underlying follicle pool.
We draw on data from a sample of recently pregnant women to extend observations on the natural history of ovarian ageing. First, we describe the relations between chronological age and several potential indicators of ovarian age: the number of antral follicles, the diameter of the largest follicle, the surface area of the follicles, ovarian volume, and levels of FSH, inhibin B and estradiol (E2). We also describe associations between indicators. Next, from among the indicators that can be easily obtained (age, ovarian volume and levels of FSH, inhibin B and E2) we identify the combination that best predicts antral follicle count. We also consider the utility of inhibin B level since, in clinical practice, measurement of this hormone is less common than measurement of FSH and E2.
Materials and methods
Participants
Selection criteria and the protocol for this study are described in detail elsewhere (Kline et al., 2004). The sample derives from a study designed to test the hypothesis that the association between advancing chronological age and trisomic pregnancy reflects an association between the size of the oocyte pool and trisomy, with risk higher in women with fewer oocytes. The data did not support the hypothesis.
The protocol
Briefly, from September 1998 to April 2001, we identified women aged ≥18 years with singleton prefetal losses (developmental age <9 weeks) whose products of conception were submitted to the Pathology Department of a hospital in New York State. We asked for permission to karyotype the abortus. If a woman's loss was successfully karyotyped, we asked her to complete a short telephone interview to determine her eligibility. Eligible women who consented to the protocol completed a more extensive telephone interview and made two visits to the study hospital during the first week of their second or later menstrual cycle, the first on day 1–4 for a blood sample and the second on day 5–7 for transvaginal sonography.
To obtain valid ovarian age measures, we required: no pituitary disorder or hormonal disorder related to ovarian function, no oophorectomy, no hormonal medication, no pregnancy at the time of ultrasound, no breastfeeding or breastfeeding no more than once per day during the menstrual cycle preceding the study assessments. We required that any diagnosis be current, the report of the diagnostic work-up informative and the clinical symptoms and treatment consistent with the diagnosis. The study reproductive endocrinologist (A.C.K.) reviewed the interview data to determine whether or not a potential participant currently had a condition associated with altered hormone levels.
Women with spontaneous abortions
Of the 244 women with karyotyped losses, 127 (52%) completed the protocol (Table I). The principal reasons for not completing the protocol were: refusal (23%) and ineligibility (25%), primarily due to use of hormonal contraceptives or pregnancy soon after the index loss. Six women were excluded because of hormonal conditions and another six were excluded due to use of fertility drugs, although only two had experienced conception delay >1 year prior to the study pregnancy.
Women who completed the protocol were on average older than women who did not. The age difference arose chiefly because younger women were more likely to begin hormonal contraception immediately after their loss. Among the 205 women who completed the eligibility interview, adjusting for age, the odds of completing the protocol did not differ with education, parity or number of prior induced abortions; completion rates were significantly higher for women with one or more prior spontaneous abortions than for women with no prior losses (74 versus 56%).
Women with live births
For each woman with a trisomic loss who completed the study, we selected an age-matched control with a chromosomally and anatomically normal live birth ≥1800 g, no pregnancy loss since the index pregnancy and no known trisomic pregnancy. They were selected from the hospital delivery log of women who delivered during the 7–13 months preceding the date of selection. Live birth controls were matched to trisomy cases for projected age (±6 months) at the sonography visit. If a selected control was ineligible for the study or refused to participate, we replaced her. The protocol for women with live births was identical to the protocol for women with prefetal losses.
In total, we selected 219 women with live births, 65 of whom (30%) completed the protocol (Table I). The principal reasons for not completing the protocol were refusal (31%) and ineligibility (37%), primarily due to use of hormonal contraceptives or breastfeeding. Two women were excluded because of hormonal conditions.
Women who completed the protocol tended to be older, though not significantly so, than women who did not. Among the 144 women who completed the eligibility interview, the odds of completing the protocol did not differ with educational attainment, parity, number of prior induced abortions or number of prior spontaneous abortions.
Analytical sample
Analyses exclude repeat study entrances of four women (all with pregnancy losses) to maintain the independence of observations. They also exclude 12 women: eight women for whom we were able to scan only one ovary—even though, based on history and transabdominal scans, each woman was known to have two ovaries—and four women who had conditions—three a cyst and one an endometrioma—which might have obscured the follicle count. Thus the analytical sample includes 176 women with both ovaries scanned and unobstructed.
Characteristics of the sample (Table II)
Among the 176 women, 117 had an index pregnancy ending in spontaneous abortion and 59 an index pregnancy ending in live birth. Average age at ultrasound was 34 years (range 22–48). The majority were white and had completed high school. Ninety-five per cent completed the blood and sonography protocols after the second or third menstrual period following the index loss or, for women with live births, the introductory letter.
Indicators of ovarian age
Antral follicles
We used four transducers over the course of the study: the first two, Acuson EXP 128, operated at a frequency of 7 MHz; the second two, Acuson Sequoia (8–10 MHz) were usually used at 10 MHz. The sonography technician scanned each ovary in transverse and longitudinal views at a subjectively constant velocity, repeating scans, as needed, until she obtained an optimal scan.
We videotaped the sonography scan. The scans were counted in four randomized batches. The randomization procedure was unknown to the sonographer.
The sonographer (M.L.R.) identified the optimal scan, converted it to a digitized format and exported it to Matrox Inspector (Matrox Electronic Systems Ltd, Dorval, Quebec, Canada 2005), an interactive imaging software. We used the software to: (i) follow each sonolucency interpreted as a follicle through the scan, frame by frame, to identify its maximum diameter; and (ii) measure the maximum diameter of each follicle in the vertical plane by calibrating measurements to the centimetre scale generated by the ultrasound machine. Diameters ranged from 1.2 to 24.1 mm, with 99.3% of follicles <12 mm in diameter.
To assess the reliability of the counting procedure, we counted again the follicles in 40 ovaries (Kline et al., 2004). The intra-class correlation coefficient was 0.92. Analyses use the second count for the 20 women whose scans were counted twice. Counts ranged from 2 to 70 (median=15, mean=18.7, SD=12.8). The mean difference between counts in the left and right ovaries was 0.2 (SD=5.4, range=−15 to 28); the correlation between them was 0.70 (P<0.0001).
We also examined associations with three alternative measures: (i) diameter of the largest follicle, because some data suggest that advancing age is associated with earlier emergence of the dominant follicle (Klein et al., 1996a, 2000); (ii) the sum of the antral follicle surface areas, to examine whether surface area is more closely related to inhibin B level than to follicle count; (iii) total ovarian volume, as a possible indicator, easily obtained, of the number of antral follicles. We assumed that antral follicles are spherical, estimating the surface area of each by 4πr2, where r is the radius. We assumed that each ovary is ellipsoidal, estimating the volume of each by D1×D2×D3× π/6, where D1, D2 and D3 are the three dimensions of the ovary. For the sum of the surface areas, the mean difference between left and right ovaries was −55.1 mm2 (SD=511.2, range −1710.6 to 1315.2); the correlation between them was 0.40 (P<0.0001). For ovarian volume, the mean difference between left and right ovaries was −0.8 cm3 (SD=4.5, range=−16.8 to 16.1); the correlation between them was 0.25 (P=0.25).
Serum hormone levels
Blood samples were processed in a refrigerated centrifuge and, after separation, sera were frozen at −25°C at the study hospital; they were shipped to New York City and stored at −20°C. FSH and E2 were measured by solid-phase chemiluminescent enzyme immunoassays (Diagnostic Products Co. Los Angeles, CA) (Immulite); dimeric inhibin B was measured by radioimmunoassay (Oxford Bio-Innovation Ltd, Upper Heyford, Oxfordshire, England). For FSH, sensitivity (the minimum detection limit) was 0.1 mIU/ml; intra- and inter-assay coefficients of variation (CV) were 9.3 and 10.5% respectively. For inhibin B, sensitivity was 20 pg/ml; intra- and inter-assay CV were 5.1 and 6.2%, respectively. For E2, sensitivity was 20 pg/ml; intra- and inter-assay CV were 1.9 and 5% respectively.
Table III shows summary statistics on each of the ovarian age indicators. Follicles were counted and hormones assayed without knowledge of any subject characteristics.
Statistical analysis
We used a logarithmic transformation for all ovarian age indicators to meet the normal error assumption of least squares regression. For antral follicle count, follicle maximum diameter and follicle surface area we used the transformation ln(1+measure) since, in theory, a woman might have no antral follicles.
We first describe the magnitude of associations: (i) between chronological age and each ovarian age indicator; and (ii) between the ovarian age indicators, adjusting for chronological age. For each indicator we illustrate the pattern of change with chronological age by showing medians and the least squares regression equation that describes change with age. We fit models with first-, second- and third-order terms for age, but retained a higher-order term only if it significantly improved the proportion of variance explained and visual inspection of the data suggested that the association with age was not log-linear. The descriptive regression equations are based on 168 women; they exclude eight women with one or two ovarian age indicators that were >3 SD from the value predicted, given age.
Second, drawing on the entire sample of 176, we used ordinary least squares regression analysis to predict follicle count using chronological age and readily obtained indicators of ovarian age—namely, ovarian volume and hormone levels.
We evaluated the accuracy of eight regression models to discriminate between women with low antral follicle count (‘older’ ovarian age) and high antral follicle count using Receiver Operating Characteristic (ROC) curves (McNeil and Hanley, 1984). We defined ‘low count’ as ≤10 antral follicles. We chose ≤10 follicles for scientific and practical reasons: it corresponds to the median count at 39 years, an age at which infecundity rates increase steeply (Schwartz and Mayaux, 1982; Menken et al., 1986); it demarcates the lowest 30% of the sample, providing a sufficient number of women with ‘low count’ for analysis.
The larger the area under the ROC curve, the better the discriminatory capability of the model. The two models with the best ROCs include measures taken at two different points during the menstrual cycle, on day 1–4 for hormones and on day 5–7 for ovarian volume. Five models, including the model with age only, which are based on data from a single time-point, might be more practicable for clinicians. For each model, we determined the cutpoint that corresponds to sensitivity of ≥80% and set out the corresponding specificity, positive predictive value and negative predictive value. Because predictive value is a function of the proportion with low count, we also describe the characteristics of five of the models separately for women aged <35 and ≥35 years.
We repeated all regression analyses adjusting for the outcome of the index pregnancy (spontaneous abortion versus live birth). Results were unchanged (data not shown). For our primary regression model predicting follicle count, we also examined whether the addition of other covariates (i.e. obstetric variables, menstrual characteristics, body mass index, education, smoking) added significantly to the proportion of variance in follicle count explained by the model; they did not.
Results
Associations with chronological age
All ovarian age indicators are significantly associated with chronological age in the expected direction (Table IV). The correlation is strongest with ln(1+antral follicle count) (r=−0.52). Correlations were moderate for ln(1+follicle maximum diameter), ln(1+follicle surface area), ln(ovarian volume) and ln(FSH), ranging from −0.26 to 0.35. Correlations were weak for ln(inhibin B) and ln(E2).
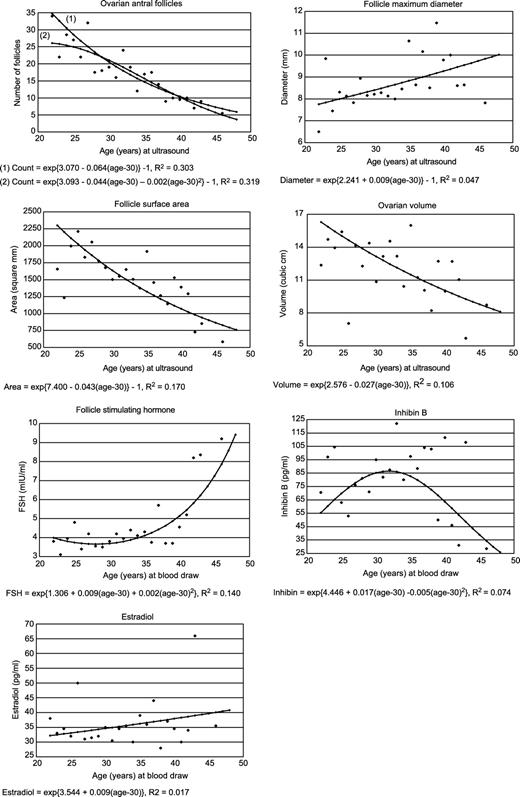
Figure 1 illustrates the shape of the relations between chronological age and each of the ovarian age indicators. For antral follicle count, we illustrate two models—one with only a linear term for age and one with both linear and quadratic age terms. The quadratic age term improved the R2 of the regression model by 1.6% (P=0.053). Follicle surface area and ovarian volume decrease approximately exponentially with age. Maximum follicle diameter and E2 increase approximately exponentially with age. For FSH, the model includes a quadratic term; levels begin to rise steeply around age 40 years. The pattern of results for inhibin B suggests that levels are either constant or increasing until the late 30s and low after age 40 years; the curve drawn here does not fit the data well.
Follicle surface area is a function of the number of follicles and their size. We used least squares regression to examine whether age was associated with follicle surface area independently of its association with follicle count. Without adjustment for follicle count, age accounts for 17.0% of the variance in ln(1+follicle surface area); with adjustment, the corresponding proportion is 0.2% (P=0.33).
Age-adjusted correlations between ovarian age indicators (Table IV)
Ln(1+antral follicle count) is most strongly related to Ln(1+follicle surface area) (r=0.76) and significantly associated with ovarian volume (r=0.48), Ln(FSH) (r=−0.34) and Ln(inhibin B) (r=0.24). Among the remaining measures, the pattern of associations is, for the most part, consistent with expectation. For example, Ln(1+follicle surface area) is positively associated with Ln(inhibin B) (r=0.34) and with Ln(1+follicle maximum diameter) (r=0.47). Ln(FSH) is inversely related to Ln(E2) (r=−0.23).
Ln(FSH) is not correlated with Ln(inhibin B) (r=−0.03). Because inhibin B levels were lower on day 1 (median=33.0 pg/ml, n=16) than on days 2–4 (median=86.5 pg/ml, n=160), we repeated the analysis, limiting the sample to women with blood samples taken on days 2–4. Ln(FSH) and Ln(inhibin B) are weakly correlated (r=−0.15, P=0.053). The correlations between inhibin B and other ovarian indicators are similar to those in the entire sample.
Predicting antral follicle count (Table V)
Using only age and four readily available measures (ovarian volume, FSH, inhibin B, E2), we sought the regression model which best predicts Ln(1+antral follicle count). Only E2 is not independently and significantly related to follicle count (R2=0.005, adjusting for chronological age, P=0.28). Thus, we did not consider E2 further in the regression analyses.
The model which includes, in addition to age, ovarian volume, FSH and inhibin B explains 49.6% of the variance in follicle count (Table V, model A). Omitting inhibin B from the model decreases the proportion of variance explained to 48.3% (model B). The standardized β values indicate that the magnitude of change, per SD unit increase, is about the same for age and Ln(1+ovarian volume) (standardized β= −0.33 and 0.35 respectively, model A), lower for Ln(FSH) (standardized β=−0.23) and lower still for Ln(inhibin B) (standardized β=0.12).
Receiver operating characteristics
Fifty-three (30.1%) women had ≤10 antral follicles. We assessed the performance of all models to distinguish women with ≤10 follicles from women with >10 (Table VI). The areas under all ROC curves are good, especially for models A (age, ovarian volume, FSH, inhibin B) and B (age, ovarian volume, FSH). For comparison, we show the model which includes only a linear term for age; the addition of a quadratic term for age did not improve the ROC curve. Using the cutpoint which produces a sensitivity of 80%, models A and B increase the positive predictive value to 58–60%, compared with 42% for a model which includes only age. Of the three models which include age plus a single ovarian age indicator, at the cutpoint corresponding to a sensitivity of 80%, the positive predictive value is highest (55%) for the model with ovarian volume.
We evaluated the performance of five models in younger (<35 years) and older (≥35 years) women separately (Table VII). We chose the two models with the best performance overall (models A and B), the model based on results from a blood test only (model D), the model based on measurement of the ovary only (model E) and the model based simply on age (model H). At the cutpoint which corresponds to a sensitivity of ≥80%, all models perform better among older women than among younger women. For example, using model A, among older women, at 80% sensitivity, specificity is 0.77, positive predictive value 0.79 and negative predictive value 0.78. The analogous values in younger women are specificity 0.39, positive predictive value 0.17 and negative predictive value 0.92. The disparities arise from the fact that predictive values are sensitive to the proportion of women with low antral count (13.9% in the 101 young women, 52.0% in the 75 older women). In both age strata, use of the best model (model B for young women, model A for older women) increases the ability to identify women with low antral count by ∼50%. For example, among older women, without information other than age we correctly identify 52% of women with low antral count; using model A we correctly identify 79%.
Discussion
Our study adds to a small body of evidence on antral follicle count among women of presumed fecundability (Reuss et al., 1996; Schipper et al., 1998) or demonstrated fertility (Erdem et al., 2002; Scheffer et al., 2003).
The strengths of the study are several. First, all participants were of demonstrated fecundability and 71% had at least one live birth, avoiding the potential biases of assisted reproduction samples. Second, all ovarian age indicators were assessed without knowledge of maternal characteristics, eliminating the possibility of bias. Third, reliability was excellent: the procedure for counting antral follicles was repeatable; the intra- and inter-assay CV for the hormone assays were high. Fourth, ovaries were imaged on day 5–7 rather than, as in previous studies, during the early follicular phase, maximizing the ability to detect developing follicles. (Earlier, follicles are often too small to see; later, the emerging dominant follicle may obscure smaller follicles.)
Three aspects of the study design are of possible concern. First, the sample over-represents women with spontaneous abortions. We do not believe this feature limits generalizability since spontaneous abortion is unrelated to these ovarian age indicators (Kline et al., 2004) and all results persisted when analyses were adjusted for the index pregnancy. Moreover, as noted above, nearly all women had at least one previous live birth. Second, because all women had conceived recently (0.3–2.5 years prior to the study sonogram, mean 0.9 years), at older ages (the late 30s and 40s) our sample may over-represent women of high fecundability. This over-representation may alter the patterns of change observed at older ages. Third, we measured hormones and counted follicles at different times during the follicular phase, an approach which may limit the clinical utility of regression equations which draw on measures from two different days.
In our sample, as expected, ln(1+antral follicle count) showed the strongest association with chronological age (r=−0.52). Age was modestly associated with ln(FSH) (r=0.35), ln(1+follicle maximum diameter) (r=0.26) and ln(ovarian volume) (r=−0.26). Age was statistically significantly associated with ln(E2) (r=0.18) and ln(inhibin B) (r=−0.16), although both correlations were small. The association of age with follicle surface area was entirely explained by follicle count.
For comparison, we consider the two largest of the aforementioned studies. Scheffer et al. (2003), in the Netherlands, recruited 162 regularly menstruating volunteers, 25–46 years; they obtained ovarian age measures on day 1–4 of the cycle. Erdem et al. (2002), in Turkey, drew on a sample of 108 menstruating women, 35–50 years, with minor gynaecological problems; all measures were made on day 3 of the cycle. The two studies differ from ours in several respects. First, both obtained follicular and ovarian measures on a single day during the early follicular phase. The range of follicle sizes in both studies was small to moderate (2–10 mm in the Netherlands, <10 mm in Turkey), whereas we counted all follicles (of which 96% were 2–10 mm). The difference in follicle size is likely a function of the timing of the measures. Second, in both studies, follicular and ovarian measures were taken during the scan, leaving open the potential for bias related to awareness of the woman's age. Data from the Netherlands (Scheffer et al., 2002), however, show both high inter-observer agreement for counts obtained during a scan and high intra-observer agreement between real-time counts and subsequent counts from stored scans. Third, both studies analysed ovarian age indicators without logarithmic transformation.
Average follicle count (our computations from published data) is ∼3.3 per woman in the Turkish sample, about nine in the Netherlands sample and 18.7 in our sample. The differences most likely reflect the higher proportions of older women and the earlier time-period of scanning in the two previous studies, when a smaller proportion of developing follicles are large enough to detect by sonography.
Observations in the Netherlands sample are roughly compatible with ours—chronological age is more strongly associated with follicle count than with FSH, inhibin B, E2 or with measures of the ovary or follicles (Scheffer et al., 2003). As in our data, chronological age is modestly associated with ovarian volume and FSH, but the correlation of age with E2 is stronger. The higher proportion of women aged >40 years in the Netherlands sample probably accounts for the somewhat higher correlations of chronological age with follicle count and E2.
In the Turkish sample (Erdem et al., 2002), chronological age is more strongly associated with FSH than with follicle count, ovarian volume or E2. Moreover, the association with E2 is inverse, rather than positive as in our study and the Netherlands study. This pattern of results probably reflects the age distribution of the sample. The inverse correlation of age with E2 was confined to women aged 45–50 years, suggesting that many, though menstruating, were close to their final menstrual periods.
There is little doubt that antral follicle count declines monotonically with age, but various models describe the shape. In our sample, the association of chronological age with ln(1+antral follicle count) is well fitted by either of two models—a simple exponential model (R2=0.30) or a model which includes both linear and quadratic terms (R2=0.32). While the quadratic age term is marginally statistically significant, it accounts for only 1.6% of the variance in count, leaving uncertain which model most fairly represents the data. The latter equation suggests that follicle count declines more steeply after about age 27 years than before. One intuitively appealing feature of the equation is the suggestion of a natural upper limit to antral follicle count. Our models contrast with those fitted by Scheffer and colleagues, who also fitted two models—a biphasic model in which ln(count) decreases more steeply beginning at age 38 years than before (Scheffer et al., 1999) and a linear model relating age to count until age 46 years (Broekmans et al., 2004). (It is beyond the scope of this paper to combine the evidence from these studies with autopsy and hysterectomy data to model the relation of age to antral follicle count.)
Age explained only a small proportion of the variance in ovarian volume and FSH in our sample. Nevertheless, our data are consistent with previous observations. Data from a large series of women enrolled in a cancer screening project show that average ovarian volume declines between ages 25 and 91 years (Pavlik et al., 2000). Like previous studies (Reyes et al., 1977; Metcalf and Livesey, 1985; Lee et al., 1988; Lenton et al., 1988; Cramer et al., 1994; Broekmans et al., 1998), our data indicate that FSH levels increase beginning around age 40 years.
With respect to inhibin B, our data indicate that the association with age is not monotonic; rather, inhibin B levels are constant or increasing until about age 40 years and then decrease. These results are compatible with two studies which show that inhibin B levels are lower in older regularly cycling women than in younger women (Klein et al., 1996b; Welt et al., 1999); at later ages (i.e. 39–52 years in one cross-sectional study), levels appear to decline linearly with age (Danforth et al., 1998).
In our sample, adjusting for age, ln(inhibin B) is modestly correlated with ln(1+follicle count) (r=0.24) and slightly more strongly correlated with ln(1+follicle surface area) (r=0.34), results consistent with those from the Netherlands sample (Scheffer et al., 2003). Taken together, the two studies suggest that inhibin B measured on day 1–4 is not a good indicator of the number of developing follicles. Since inhibin B is produced by the developing cohort of antral follicles (Groome et al., 1996; Burger, 2000), measurements later in the follicular phase may more accurately reflect the size and quality of the developing cohort.
Our data show only a weak positive relation between age and E2. This result joins an already inconsistent body of evidence, among menstruating women, showing a positive association (Welt et al., 1999; Scheffer et al., 2003), no association (Klein et al., 1996b) or an inverse association (Erdem et al., 2002). This inconsistency is probably explained by methodological disparities.
Clinical application
From among the easily obtained indicators—age, ovarian volume and levels of FSH, inhibin B and E2—we identified the combination that best predicts ln(1+antral follicle count). Other informative hormones might also be easy to obtain and useful. For example, two recent studies—one in presumably fecund women (de Vet et al., 2002) and the other in infertile women (van Rooij et al., 2002)—suggest that anti-Müllerian hormone levels may be a useful predictor of antral follicle count.
Of the five indicators examined, only E2 was not significantly related to ln(1+follicle count). The regression model which included age, ovarian volume, FSH and inhibin B explained 49.6% of the variance in count. Age and ovarian volume were the strongest predictors, followed by FSH. The association between ovarian volume and count is not surprising, since the ovary enlarges to accommodate the developing follicles. Inhibin B, though significantly associated with count, was responsible for only a small proportion of its variance. Removing this term from the equation decreased the proportion of variance explained only trivially, to 48.3%.
To predict which women have ≤10 antral follicles, models that include ovarian age indicators as well as chronological age improve markedly on the model that includes chronological age alone. Two models—A (chronological age, ovarian volume, FSH and inhibin B) and B (chronological age, ovarian volume and FSH)—are virtually indistinguishable in their ability to discriminate between women with low and high count. For the clinician, these models have the disadvantage that they require measures of both ovarian volume and hormones. Moreover, our regression equation derives from ovarian and hormone data which were collected on two different days of the cycle. Model D, which includes only chronological age and hormone levels, performs nearly as well as models A and B.
All models performed less well in younger (<35 years) women than in older (≥35 years) women, as expected given the prevalence (13.9%) of low antral follicle count among younger women. For a presumably fecund young woman who wants to defer childbearing, our models do not improve upon knowledge of her age alone for predicting whether or not she will encounter problems when she later tries to conceive. For an older woman who wants to know how long she can postpone childbearing or who is trying to conceive and wants to know whether to expect difficulties, our models improve upon prediction based on chronological age alone. In our sample, in which 52% of older women had low count, the best model has a positive predictive value of 79%, a marked improvement over the positive predictive value (60%) of the model based only on chronological age. Thus, our models help to identify women who might benefit from expedited evaluation (for example, actually counting follicles).
Three caveats are in order. First, our predictive regression equations, which derive from a single sample, need replication to determine validity. Second, strictly speaking our equations apply to women of demonstrated (or presumed) fecundability; it remains to test whether or not they are useful to women seeking treatment for infertility. Third, an underlying assumption for this work is that antral follicle count predicts fecundability. While it is generally thought that low count is associated with low fecundability, data are limited to women seeking care for infertility and their response to assisted reproduction treatment. These data do not readily translate into predictions about conception for women of known or presumed fecundability, nor are they generalizable. It remains to test this assumption in samples not selected for difficulty conceiving.
Finally, one aspect of the analytical approach merits elaboration. Although we defined low count as ≤10 antral follicles, the choice was not guided by empirical evidence; the biologically relevant cutpoint may be lower. In our sample, antral follicle count of 10 corresponds to the median at age 39 years—when the risk of infecundity is ≥20% (Menken et al., 1986) and the proportion of women taking >1 year to conceive may be on the order of 50% (Schwartz and Mayaux, 1982). If our equations are valid, they will improve the prediction of time to conception in older women beyond that provided by chronological age alone.
Chronological age and indicators of ovarian age: Medians and fitted regression equations. Medians are based on 176 women. The regression equations are based on 168 women; they exclude eight women with one or two ovarian age indicators that were >3 SD from that predicted, given age.
Number of women identified/selected, declining the study or ineligible, or completing the protocol
| . | Prefetal loss . | Live birth . | ||
|---|---|---|---|---|
| Identified/selected | 435 | 219 | ||
| Tissue culture set up | 269 | NA | ||
| Karyotyped | 244 | NA | ||
| Moved or not located | 1 | 7 | ||
| Declined eligibility interview | 36 | 56 | ||
| Eligible, declined study or lost during follow-up | 19 | 11 | ||
| Ineligiblea | 61 | 80 | ||
| Hormonal contraceptiveb | 25 | 38 | ||
| Pregnantc | 18 | 12 | ||
| Breastfeeding | 3 | 23 | ||
| Oophorectomy | 1 | 3 | ||
| Other condition affecting ovaries or hormonesd | 6 | 2 | ||
| Fertility medication | 6 | 0 | ||
| Other hormonal medicatione | 1 | 1 | ||
| Otherf | 1 | 1 | ||
| Completed protocol | 127 | 65 | ||
| Analytical exclusions | ||||
| Repeat entranceg | 4 | 0 | ||
| Only one informative ovary | 6 | 6 | ||
| Analytical sample | 117 | 59 | ||
| . | Prefetal loss . | Live birth . | ||
|---|---|---|---|---|
| Identified/selected | 435 | 219 | ||
| Tissue culture set up | 269 | NA | ||
| Karyotyped | 244 | NA | ||
| Moved or not located | 1 | 7 | ||
| Declined eligibility interview | 36 | 56 | ||
| Eligible, declined study or lost during follow-up | 19 | 11 | ||
| Ineligiblea | 61 | 80 | ||
| Hormonal contraceptiveb | 25 | 38 | ||
| Pregnantc | 18 | 12 | ||
| Breastfeeding | 3 | 23 | ||
| Oophorectomy | 1 | 3 | ||
| Other condition affecting ovaries or hormonesd | 6 | 2 | ||
| Fertility medication | 6 | 0 | ||
| Other hormonal medicatione | 1 | 1 | ||
| Otherf | 1 | 1 | ||
| Completed protocol | 127 | 65 | ||
| Analytical exclusions | ||||
| Repeat entranceg | 4 | 0 | ||
| Only one informative ovary | 6 | 6 | ||
| Analytical sample | 117 | 59 | ||
Includes 14 women (two with prefetal losses, 12 with live births) who provided information on their eligibility status but declined the eligibility interview.
Sixty-one women were taking oral contraceptives; two live birth controls had intrauterine devices that included a hormonal component. Two live birth controls were also breastfeeding.
One live birth control was also breastfeeding.
Five women (four with prefetal losses, one live birth control) had been diagnosed with polycystic ovary disease, two women (one with a prefetal loss, one live birth control) with hyperprolactaemia, and one woman with a prefetal loss had been diagnosed with anovulation.
One woman with a prefetal loss was taking oral contraceptives to regulate her periods (oligomenorrhoea, possible polycystic ovary disease). One live birth control was taking progesterone ‘for a possible cyst’.
One woman with a prefetal loss did not complete the protocol within 8 months of her spontaneous abortion. One live birth control had had a previous trisomic conception.
To maintain the independence of observations, the analysis excludes repeat study entrances of four women.
Number of women identified/selected, declining the study or ineligible, or completing the protocol
| . | Prefetal loss . | Live birth . | ||
|---|---|---|---|---|
| Identified/selected | 435 | 219 | ||
| Tissue culture set up | 269 | NA | ||
| Karyotyped | 244 | NA | ||
| Moved or not located | 1 | 7 | ||
| Declined eligibility interview | 36 | 56 | ||
| Eligible, declined study or lost during follow-up | 19 | 11 | ||
| Ineligiblea | 61 | 80 | ||
| Hormonal contraceptiveb | 25 | 38 | ||
| Pregnantc | 18 | 12 | ||
| Breastfeeding | 3 | 23 | ||
| Oophorectomy | 1 | 3 | ||
| Other condition affecting ovaries or hormonesd | 6 | 2 | ||
| Fertility medication | 6 | 0 | ||
| Other hormonal medicatione | 1 | 1 | ||
| Otherf | 1 | 1 | ||
| Completed protocol | 127 | 65 | ||
| Analytical exclusions | ||||
| Repeat entranceg | 4 | 0 | ||
| Only one informative ovary | 6 | 6 | ||
| Analytical sample | 117 | 59 | ||
| . | Prefetal loss . | Live birth . | ||
|---|---|---|---|---|
| Identified/selected | 435 | 219 | ||
| Tissue culture set up | 269 | NA | ||
| Karyotyped | 244 | NA | ||
| Moved or not located | 1 | 7 | ||
| Declined eligibility interview | 36 | 56 | ||
| Eligible, declined study or lost during follow-up | 19 | 11 | ||
| Ineligiblea | 61 | 80 | ||
| Hormonal contraceptiveb | 25 | 38 | ||
| Pregnantc | 18 | 12 | ||
| Breastfeeding | 3 | 23 | ||
| Oophorectomy | 1 | 3 | ||
| Other condition affecting ovaries or hormonesd | 6 | 2 | ||
| Fertility medication | 6 | 0 | ||
| Other hormonal medicatione | 1 | 1 | ||
| Otherf | 1 | 1 | ||
| Completed protocol | 127 | 65 | ||
| Analytical exclusions | ||||
| Repeat entranceg | 4 | 0 | ||
| Only one informative ovary | 6 | 6 | ||
| Analytical sample | 117 | 59 | ||
Includes 14 women (two with prefetal losses, 12 with live births) who provided information on their eligibility status but declined the eligibility interview.
Sixty-one women were taking oral contraceptives; two live birth controls had intrauterine devices that included a hormonal component. Two live birth controls were also breastfeeding.
One live birth control was also breastfeeding.
Five women (four with prefetal losses, one live birth control) had been diagnosed with polycystic ovary disease, two women (one with a prefetal loss, one live birth control) with hyperprolactaemia, and one woman with a prefetal loss had been diagnosed with anovulation.
One woman with a prefetal loss was taking oral contraceptives to regulate her periods (oligomenorrhoea, possible polycystic ovary disease). One live birth control was taking progesterone ‘for a possible cyst’.
One woman with a prefetal loss did not complete the protocol within 8 months of her spontaneous abortion. One live birth control had had a previous trisomic conception.
To maintain the independence of observations, the analysis excludes repeat study entrances of four women.
Selected characteristics of women who completed the study
| No. of women | 176 | |
| Outcome of the index pregnancy | ||
| Spontaneous abortiona | 117 | |
| Live birth | 59 | |
| Age at ultrasound, mean (SD) | 34.0 (5.4) | |
| No. of live births, mean (SD) | 1.3 (1.1) | |
| No. of spontaneous abortionsb , mean (SD) | 1.2 (1.1) | |
| No. of induced abortions, mean (SD) | 0.2 (0.5) | |
| Percentage distributions | ||
| Education (%) | ||
| ≤high school | 19.3 | |
| Some college | 29.5 | |
| College graduate | 31.3 | |
| Postgraduate degree | 19.9 | |
| Ethnicity (%) | ||
| White, non-Hispanic | 94.3 | |
| Menstrual period preceding study protocol (%) | ||
| Period 2 | 74.4 | |
| Period 3c | 20.5 | |
| Period 4–7 | 5.1 | |
| Day of blood sampling | ||
| Day 1 | 9.1 | |
| Day 2 | 31.3 | |
| Day 3 | 33.0 | |
| Day 4 | 26.7 | |
| Day of sonography | ||
| Day 5 | 33.5 | |
| Day 6 | 32.4 | |
| Day 7 | 34.1 | |
| No. of women | 176 | |
| Outcome of the index pregnancy | ||
| Spontaneous abortiona | 117 | |
| Live birth | 59 | |
| Age at ultrasound, mean (SD) | 34.0 (5.4) | |
| No. of live births, mean (SD) | 1.3 (1.1) | |
| No. of spontaneous abortionsb , mean (SD) | 1.2 (1.1) | |
| No. of induced abortions, mean (SD) | 0.2 (0.5) | |
| Percentage distributions | ||
| Education (%) | ||
| ≤high school | 19.3 | |
| Some college | 29.5 | |
| College graduate | 31.3 | |
| Postgraduate degree | 19.9 | |
| Ethnicity (%) | ||
| White, non-Hispanic | 94.3 | |
| Menstrual period preceding study protocol (%) | ||
| Period 2 | 74.4 | |
| Period 3c | 20.5 | |
| Period 4–7 | 5.1 | |
| Day of blood sampling | ||
| Day 1 | 9.1 | |
| Day 2 | 31.3 | |
| Day 3 | 33.0 | |
| Day 4 | 26.7 | |
| Day of sonography | ||
| Day 5 | 33.5 | |
| Day 6 | 32.4 | |
| Day 7 | 34.1 | |
Karyotypes of the abortus: trisomy (n=52), other chromosomally abnormal (n=22), confirmed chromosomally normal (n=21), unconfirmed chromosomally normal female (n=22). We classify as ‘unconfirmed chromosomally normally female’ those losses in which the karyotype was 46,XX and confirmatory DNA analysis was either not possible because of inadequate specimens (n=17) or DNA analysis indicated inadvertent karyotyping of maternal cells (n=5).
Excludes one woman with unknown number of spontaneous abortions.
Includes two women where the menstrual period preceding the study protocol may have been either period 2 or period 3.
Selected characteristics of women who completed the study
| No. of women | 176 | |
| Outcome of the index pregnancy | ||
| Spontaneous abortiona | 117 | |
| Live birth | 59 | |
| Age at ultrasound, mean (SD) | 34.0 (5.4) | |
| No. of live births, mean (SD) | 1.3 (1.1) | |
| No. of spontaneous abortionsb , mean (SD) | 1.2 (1.1) | |
| No. of induced abortions, mean (SD) | 0.2 (0.5) | |
| Percentage distributions | ||
| Education (%) | ||
| ≤high school | 19.3 | |
| Some college | 29.5 | |
| College graduate | 31.3 | |
| Postgraduate degree | 19.9 | |
| Ethnicity (%) | ||
| White, non-Hispanic | 94.3 | |
| Menstrual period preceding study protocol (%) | ||
| Period 2 | 74.4 | |
| Period 3c | 20.5 | |
| Period 4–7 | 5.1 | |
| Day of blood sampling | ||
| Day 1 | 9.1 | |
| Day 2 | 31.3 | |
| Day 3 | 33.0 | |
| Day 4 | 26.7 | |
| Day of sonography | ||
| Day 5 | 33.5 | |
| Day 6 | 32.4 | |
| Day 7 | 34.1 | |
| No. of women | 176 | |
| Outcome of the index pregnancy | ||
| Spontaneous abortiona | 117 | |
| Live birth | 59 | |
| Age at ultrasound, mean (SD) | 34.0 (5.4) | |
| No. of live births, mean (SD) | 1.3 (1.1) | |
| No. of spontaneous abortionsb , mean (SD) | 1.2 (1.1) | |
| No. of induced abortions, mean (SD) | 0.2 (0.5) | |
| Percentage distributions | ||
| Education (%) | ||
| ≤high school | 19.3 | |
| Some college | 29.5 | |
| College graduate | 31.3 | |
| Postgraduate degree | 19.9 | |
| Ethnicity (%) | ||
| White, non-Hispanic | 94.3 | |
| Menstrual period preceding study protocol (%) | ||
| Period 2 | 74.4 | |
| Period 3c | 20.5 | |
| Period 4–7 | 5.1 | |
| Day of blood sampling | ||
| Day 1 | 9.1 | |
| Day 2 | 31.3 | |
| Day 3 | 33.0 | |
| Day 4 | 26.7 | |
| Day of sonography | ||
| Day 5 | 33.5 | |
| Day 6 | 32.4 | |
| Day 7 | 34.1 | |
Karyotypes of the abortus: trisomy (n=52), other chromosomally abnormal (n=22), confirmed chromosomally normal (n=21), unconfirmed chromosomally normal female (n=22). We classify as ‘unconfirmed chromosomally normally female’ those losses in which the karyotype was 46,XX and confirmatory DNA analysis was either not possible because of inadequate specimens (n=17) or DNA analysis indicated inadvertent karyotyping of maternal cells (n=5).
Excludes one woman with unknown number of spontaneous abortions.
Includes two women where the menstrual period preceding the study protocol may have been either period 2 or period 3.
Summary statistics for antral follicle count, maximum follicle diameter, surface area of the follicles, ovarian volume, inhibin B, FSH and estradiol (E2) in 176 women
| . | Median . | Mean (SD) . | Geometric meana . | Range . | Percentage below assay detection level . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follicles | ||||||||||
| Count | 15.0 | 18.7 (12.8) | 15.3 | 2–70 | ||||||
| Maximum diameter (mm) | 8.5 | 9.2 (2.8) | 8.3 | 5.1–24.1 | ||||||
| Surface area (mm2) | 1459.2 | 1583.8 (781.3) | 1376.0 | 155.6–4016.6 | ||||||
| Ovary | ||||||||||
| Total volume (cm3) | 12.1 | 13.1 (5.8) | 12.2 | 2.7–33.1 | ||||||
| Hormones | ||||||||||
| FSH (mIU/ml) | 4.4 | 4.8 (2.6) | 4.2 | 0.7–16.3 | 0.0 | |||||
| Inhibin B (pg/ml) | 80.5 | 89.5 (53.8) | 72.9 | 20–272 | 9.1 | |||||
| E2 (pg/ml) | 35.0 | 40.4 (23.0) | 36.6 | 20–206 | 5.7 | |||||
| . | Median . | Mean (SD) . | Geometric meana . | Range . | Percentage below assay detection level . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follicles | ||||||||||
| Count | 15.0 | 18.7 (12.8) | 15.3 | 2–70 | ||||||
| Maximum diameter (mm) | 8.5 | 9.2 (2.8) | 8.3 | 5.1–24.1 | ||||||
| Surface area (mm2) | 1459.2 | 1583.8 (781.3) | 1376.0 | 155.6–4016.6 | ||||||
| Ovary | ||||||||||
| Total volume (cm3) | 12.1 | 13.1 (5.8) | 12.2 | 2.7–33.1 | ||||||
| Hormones | ||||||||||
| FSH (mIU/ml) | 4.4 | 4.8 (2.6) | 4.2 | 0.7–16.3 | 0.0 | |||||
| Inhibin B (pg/ml) | 80.5 | 89.5 (53.8) | 72.9 | 20–272 | 9.1 | |||||
| E2 (pg/ml) | 35.0 | 40.4 (23.0) | 36.6 | 20–206 | 5.7 | |||||
To meet the normality assumptions of ordinary least squares regression, we used natural logarithmic transformations for each ovarian age indicator. For follicle count, follicle maximum diameter and follicle surface area, where values of zero are theoretically possible, we used the transformation ln(1+follicle measure). For other ovarian age indicators we used ln(other measure). Values are reported in the original scale using the inverse transformation [exp(mean) − 1 for follicle measures and exp(mean) for other measures].
Summary statistics for antral follicle count, maximum follicle diameter, surface area of the follicles, ovarian volume, inhibin B, FSH and estradiol (E2) in 176 women
| . | Median . | Mean (SD) . | Geometric meana . | Range . | Percentage below assay detection level . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follicles | ||||||||||
| Count | 15.0 | 18.7 (12.8) | 15.3 | 2–70 | ||||||
| Maximum diameter (mm) | 8.5 | 9.2 (2.8) | 8.3 | 5.1–24.1 | ||||||
| Surface area (mm2) | 1459.2 | 1583.8 (781.3) | 1376.0 | 155.6–4016.6 | ||||||
| Ovary | ||||||||||
| Total volume (cm3) | 12.1 | 13.1 (5.8) | 12.2 | 2.7–33.1 | ||||||
| Hormones | ||||||||||
| FSH (mIU/ml) | 4.4 | 4.8 (2.6) | 4.2 | 0.7–16.3 | 0.0 | |||||
| Inhibin B (pg/ml) | 80.5 | 89.5 (53.8) | 72.9 | 20–272 | 9.1 | |||||
| E2 (pg/ml) | 35.0 | 40.4 (23.0) | 36.6 | 20–206 | 5.7 | |||||
| . | Median . | Mean (SD) . | Geometric meana . | Range . | Percentage below assay detection level . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follicles | ||||||||||
| Count | 15.0 | 18.7 (12.8) | 15.3 | 2–70 | ||||||
| Maximum diameter (mm) | 8.5 | 9.2 (2.8) | 8.3 | 5.1–24.1 | ||||||
| Surface area (mm2) | 1459.2 | 1583.8 (781.3) | 1376.0 | 155.6–4016.6 | ||||||
| Ovary | ||||||||||
| Total volume (cm3) | 12.1 | 13.1 (5.8) | 12.2 | 2.7–33.1 | ||||||
| Hormones | ||||||||||
| FSH (mIU/ml) | 4.4 | 4.8 (2.6) | 4.2 | 0.7–16.3 | 0.0 | |||||
| Inhibin B (pg/ml) | 80.5 | 89.5 (53.8) | 72.9 | 20–272 | 9.1 | |||||
| E2 (pg/ml) | 35.0 | 40.4 (23.0) | 36.6 | 20–206 | 5.7 | |||||
To meet the normality assumptions of ordinary least squares regression, we used natural logarithmic transformations for each ovarian age indicator. For follicle count, follicle maximum diameter and follicle surface area, where values of zero are theoretically possible, we used the transformation ln(1+follicle measure). For other ovarian age indicators we used ln(other measure). Values are reported in the original scale using the inverse transformation [exp(mean) − 1 for follicle measures and exp(mean) for other measures].
Correlations between chronological age and ovarian age indicatorsa with adjustment for chronological age in 176 women
| . | Age (years) . | Adjusted for chronological age . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Follicle count . | Follicle maximum diameter . | Follicle surface area . | Total ovarian volume . | FSH (mIU/ml) . | Inhibin B (pg/ml) . | E2 (pg/ml) . | ||||||
| Follicle count | −0.52¶ | 1.00 | −0.06 | 0.76¶ | 0.48¶ | −0.34¶ | 0.24† | 0.08 | ||||||
| Follicle maximum diameter | 0.26§ | 1.00 | 0.47¶ | 0.37¶ | 0.14 | 0.21** | 0.06 | |||||||
| Follicle surface area | −0.34¶ | 1.00 | 0.67¶ | −0.29§ | 0.34¶ | 0.14 | ||||||||
| Total ovarian volume | −0.26§ | 1.00 | −0.23† | 0.24† | 0.18* | |||||||||
| FSH (mIU/ml) | 0.35¶ | 1.00 | −0.03 | −0.23† | ||||||||||
| Inhibin B (pg/ml) | −0.16* | 1.00 | 0.06 | |||||||||||
| E2 (pg/ml) | 0.18* | 1.00 | ||||||||||||
| . | Age (years) . | Adjusted for chronological age . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Follicle count . | Follicle maximum diameter . | Follicle surface area . | Total ovarian volume . | FSH (mIU/ml) . | Inhibin B (pg/ml) . | E2 (pg/ml) . | ||||||
| Follicle count | −0.52¶ | 1.00 | −0.06 | 0.76¶ | 0.48¶ | −0.34¶ | 0.24† | 0.08 | ||||||
| Follicle maximum diameter | 0.26§ | 1.00 | 0.47¶ | 0.37¶ | 0.14 | 0.21** | 0.06 | |||||||
| Follicle surface area | −0.34¶ | 1.00 | 0.67¶ | −0.29§ | 0.34¶ | 0.14 | ||||||||
| Total ovarian volume | −0.26§ | 1.00 | −0.23† | 0.24† | 0.18* | |||||||||
| FSH (mIU/ml) | 0.35¶ | 1.00 | −0.03 | −0.23† | ||||||||||
| Inhibin B (pg/ml) | −0.16* | 1.00 | 0.06 | |||||||||||
| E2 (pg/ml) | 0.18* | 1.00 | ||||||||||||
To meet the normality assumptions of ordinary least squares regression, we used natural logarithmic transformations for each ovarian age indicator. For follicle count, follicle maximum diameter and follicle surface area, where values of zero are theoretically possible, we used the transformation ln(1+indicator). For ovarian volume and hormones, we used ln(indicator).
*P<0.05.
**P<0.01.
†P<0.005.
§P<0.0005.
¶P<0.0001.
Correlations between chronological age and ovarian age indicatorsa with adjustment for chronological age in 176 women
| . | Age (years) . | Adjusted for chronological age . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Follicle count . | Follicle maximum diameter . | Follicle surface area . | Total ovarian volume . | FSH (mIU/ml) . | Inhibin B (pg/ml) . | E2 (pg/ml) . | ||||||
| Follicle count | −0.52¶ | 1.00 | −0.06 | 0.76¶ | 0.48¶ | −0.34¶ | 0.24† | 0.08 | ||||||
| Follicle maximum diameter | 0.26§ | 1.00 | 0.47¶ | 0.37¶ | 0.14 | 0.21** | 0.06 | |||||||
| Follicle surface area | −0.34¶ | 1.00 | 0.67¶ | −0.29§ | 0.34¶ | 0.14 | ||||||||
| Total ovarian volume | −0.26§ | 1.00 | −0.23† | 0.24† | 0.18* | |||||||||
| FSH (mIU/ml) | 0.35¶ | 1.00 | −0.03 | −0.23† | ||||||||||
| Inhibin B (pg/ml) | −0.16* | 1.00 | 0.06 | |||||||||||
| E2 (pg/ml) | 0.18* | 1.00 | ||||||||||||
| . | Age (years) . | Adjusted for chronological age . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Follicle count . | Follicle maximum diameter . | Follicle surface area . | Total ovarian volume . | FSH (mIU/ml) . | Inhibin B (pg/ml) . | E2 (pg/ml) . | ||||||
| Follicle count | −0.52¶ | 1.00 | −0.06 | 0.76¶ | 0.48¶ | −0.34¶ | 0.24† | 0.08 | ||||||
| Follicle maximum diameter | 0.26§ | 1.00 | 0.47¶ | 0.37¶ | 0.14 | 0.21** | 0.06 | |||||||
| Follicle surface area | −0.34¶ | 1.00 | 0.67¶ | −0.29§ | 0.34¶ | 0.14 | ||||||||
| Total ovarian volume | −0.26§ | 1.00 | −0.23† | 0.24† | 0.18* | |||||||||
| FSH (mIU/ml) | 0.35¶ | 1.00 | −0.03 | −0.23† | ||||||||||
| Inhibin B (pg/ml) | −0.16* | 1.00 | 0.06 | |||||||||||
| E2 (pg/ml) | 0.18* | 1.00 | ||||||||||||
To meet the normality assumptions of ordinary least squares regression, we used natural logarithmic transformations for each ovarian age indicator. For follicle count, follicle maximum diameter and follicle surface area, where values of zero are theoretically possible, we used the transformation ln(1+indicator). For ovarian volume and hormones, we used ln(indicator).
*P<0.05.
**P<0.01.
†P<0.005.
§P<0.0005.
¶P<0.0001.
| Age (years) | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44–48 |
| n | 2 | 3 | 2 | 5 | 1 | 11 | 4 | 11 | 19 | 14 | 10 | 12 | 7 | 10 | 10 | 15 | 9 | 5 | 4 | 9 | 7 | 2 | 4 |
| Age (years) | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44–48 |
| n | 2 | 3 | 2 | 5 | 1 | 11 | 4 | 11 | 19 | 14 | 10 | 12 | 7 | 10 | 10 | 15 | 9 | 5 | 4 | 9 | 7 | 2 | 4 |
| Age (years) | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44–48 |
| n | 2 | 3 | 2 | 5 | 1 | 11 | 4 | 11 | 19 | 14 | 10 | 12 | 7 | 10 | 10 | 15 | 9 | 5 | 4 | 9 | 7 | 2 | 4 |
| Age (years) | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44–48 |
| n | 2 | 3 | 2 | 5 | 1 | 11 | 4 | 11 | 19 | 14 | 10 | 12 | 7 | 10 | 10 | 15 | 9 | 5 | 4 | 9 | 7 | 2 | 4 |
Regression models predicting antral follicle count using chronological age and three ovarian age indicators in 176 women
| Model . | β . | SE . | Standardized β . | P . | R2 . |
|---|---|---|---|---|---|
| (A) Age, volume, FSH, inhibin B | 0.496 | ||||
| Intercept | 1.702 | 0.308 | |||
| Age−30a | −0.038 | 0.007 | −0.329 | <0.0001 | |
| Ln(ovarian volume) | −0.487 | 0.083 | 0.350 | <0.0001 | |
| Ln(FSH) | −0.301 | 0.079 | −0.228 | 0.0002 | |
| Ln(inhibin B) | 0.109 | 0.052 | 0.120 | 0.0360 | |
| (B) Age, volume, FSH | 0.483 | ||||
| Intercept | 2.070 | 0.257 | |||
| Age−30 | −0.040 | 0.007 | −0.342 | <0.0001 | |
| Ln(ovarian volume) | −0.529 | 0.081 | 0.379 | <0.0001 | |
| Ln(FSH) | −0.297 | 0.079 | −0.225 | 0.0002 | |
| (C) Age, volume, inhibin B | 0.453 | ||||
| Intercept | 1.139 | 0.281 | |||
| Age−30 | −0.046 | 0.007 | −0.396 | <0.0001 | |
| Ln(ovarian volume) | 0.559 | 0.084 | 0.401 | <0.0001 | |
| Ln(inhibin B) | 0.105 | 0.054 | 0.115 | 0.0520 | |
| (D) Age, FSH, inhibin B | 0.394 | ||||
| Intercept | 2.776 | 0.271 | |||
| Age−30 | −0.044 | 0.007 | −0.378 | <0.0001 | |
| Ln(FSH) | −0.406 | 0.084 | −0.307 | <0.0001 | |
| Ln(inhibin B) | 0.181 | 0.055 | 0.199 | 0.0012 | |
| (E) Age, volume | 0.441 | ||||
| Intercept | 1.499 | 0.215 | |||
| Age−30 | −0.047 | 0.007 | −0.408 | <0.0001 | |
| Ln(ovarian volume) | −0.598 | 0.082 | 0.429 | <0.0001 | |
| (F) Age, FSH | 0.356 | ||||
| Intercept | 3.582 | 0.123 | |||
| Age−30 | −0.047 | 0.008 | −0.408 | <0.0001 | |
| Ln(FSH) | −0.415 | 0.086 | −0.314 | <0.0001 | |
| (G) Age, inhibin B | 0.312 | ||||
| Intercept | 2.201 | 0.260 | |||
| Age−30 | −0.056 | 0.007 | −0.485 | <0.0001 | |
| Ln(inhibin B) | 0.190 | 0.058 | 0.208 | 0.0013 | |
| (H) Age | 0.269 | ||||
| Intercept | 3.033 | 0.050 | |||
| Age−30 | −0.060 | 0.008 | −0.529 | <0.0001 |
| Model . | β . | SE . | Standardized β . | P . | R2 . |
|---|---|---|---|---|---|
| (A) Age, volume, FSH, inhibin B | 0.496 | ||||
| Intercept | 1.702 | 0.308 | |||
| Age−30a | −0.038 | 0.007 | −0.329 | <0.0001 | |
| Ln(ovarian volume) | −0.487 | 0.083 | 0.350 | <0.0001 | |
| Ln(FSH) | −0.301 | 0.079 | −0.228 | 0.0002 | |
| Ln(inhibin B) | 0.109 | 0.052 | 0.120 | 0.0360 | |
| (B) Age, volume, FSH | 0.483 | ||||
| Intercept | 2.070 | 0.257 | |||
| Age−30 | −0.040 | 0.007 | −0.342 | <0.0001 | |
| Ln(ovarian volume) | −0.529 | 0.081 | 0.379 | <0.0001 | |
| Ln(FSH) | −0.297 | 0.079 | −0.225 | 0.0002 | |
| (C) Age, volume, inhibin B | 0.453 | ||||
| Intercept | 1.139 | 0.281 | |||
| Age−30 | −0.046 | 0.007 | −0.396 | <0.0001 | |
| Ln(ovarian volume) | 0.559 | 0.084 | 0.401 | <0.0001 | |
| Ln(inhibin B) | 0.105 | 0.054 | 0.115 | 0.0520 | |
| (D) Age, FSH, inhibin B | 0.394 | ||||
| Intercept | 2.776 | 0.271 | |||
| Age−30 | −0.044 | 0.007 | −0.378 | <0.0001 | |
| Ln(FSH) | −0.406 | 0.084 | −0.307 | <0.0001 | |
| Ln(inhibin B) | 0.181 | 0.055 | 0.199 | 0.0012 | |
| (E) Age, volume | 0.441 | ||||
| Intercept | 1.499 | 0.215 | |||
| Age−30 | −0.047 | 0.007 | −0.408 | <0.0001 | |
| Ln(ovarian volume) | −0.598 | 0.082 | 0.429 | <0.0001 | |
| (F) Age, FSH | 0.356 | ||||
| Intercept | 3.582 | 0.123 | |||
| Age−30 | −0.047 | 0.008 | −0.408 | <0.0001 | |
| Ln(FSH) | −0.415 | 0.086 | −0.314 | <0.0001 | |
| (G) Age, inhibin B | 0.312 | ||||
| Intercept | 2.201 | 0.260 | |||
| Age−30 | −0.056 | 0.007 | −0.485 | <0.0001 | |
| Ln(inhibin B) | 0.190 | 0.058 | 0.208 | 0.0013 | |
| (H) Age | 0.269 | ||||
| Intercept | 3.033 | 0.050 | |||
| Age−30 | −0.060 | 0.008 | −0.529 | <0.0001 |
We centered the age variable by subtracting 30 from each woman’s age in years at ultrasound.
Regression models predicting antral follicle count using chronological age and three ovarian age indicators in 176 women
| Model . | β . | SE . | Standardized β . | P . | R2 . |
|---|---|---|---|---|---|
| (A) Age, volume, FSH, inhibin B | 0.496 | ||||
| Intercept | 1.702 | 0.308 | |||
| Age−30a | −0.038 | 0.007 | −0.329 | <0.0001 | |
| Ln(ovarian volume) | −0.487 | 0.083 | 0.350 | <0.0001 | |
| Ln(FSH) | −0.301 | 0.079 | −0.228 | 0.0002 | |
| Ln(inhibin B) | 0.109 | 0.052 | 0.120 | 0.0360 | |
| (B) Age, volume, FSH | 0.483 | ||||
| Intercept | 2.070 | 0.257 | |||
| Age−30 | −0.040 | 0.007 | −0.342 | <0.0001 | |
| Ln(ovarian volume) | −0.529 | 0.081 | 0.379 | <0.0001 | |
| Ln(FSH) | −0.297 | 0.079 | −0.225 | 0.0002 | |
| (C) Age, volume, inhibin B | 0.453 | ||||
| Intercept | 1.139 | 0.281 | |||
| Age−30 | −0.046 | 0.007 | −0.396 | <0.0001 | |
| Ln(ovarian volume) | 0.559 | 0.084 | 0.401 | <0.0001 | |
| Ln(inhibin B) | 0.105 | 0.054 | 0.115 | 0.0520 | |
| (D) Age, FSH, inhibin B | 0.394 | ||||
| Intercept | 2.776 | 0.271 | |||
| Age−30 | −0.044 | 0.007 | −0.378 | <0.0001 | |
| Ln(FSH) | −0.406 | 0.084 | −0.307 | <0.0001 | |
| Ln(inhibin B) | 0.181 | 0.055 | 0.199 | 0.0012 | |
| (E) Age, volume | 0.441 | ||||
| Intercept | 1.499 | 0.215 | |||
| Age−30 | −0.047 | 0.007 | −0.408 | <0.0001 | |
| Ln(ovarian volume) | −0.598 | 0.082 | 0.429 | <0.0001 | |
| (F) Age, FSH | 0.356 | ||||
| Intercept | 3.582 | 0.123 | |||
| Age−30 | −0.047 | 0.008 | −0.408 | <0.0001 | |
| Ln(FSH) | −0.415 | 0.086 | −0.314 | <0.0001 | |
| (G) Age, inhibin B | 0.312 | ||||
| Intercept | 2.201 | 0.260 | |||
| Age−30 | −0.056 | 0.007 | −0.485 | <0.0001 | |
| Ln(inhibin B) | 0.190 | 0.058 | 0.208 | 0.0013 | |
| (H) Age | 0.269 | ||||
| Intercept | 3.033 | 0.050 | |||
| Age−30 | −0.060 | 0.008 | −0.529 | <0.0001 |
| Model . | β . | SE . | Standardized β . | P . | R2 . |
|---|---|---|---|---|---|
| (A) Age, volume, FSH, inhibin B | 0.496 | ||||
| Intercept | 1.702 | 0.308 | |||
| Age−30a | −0.038 | 0.007 | −0.329 | <0.0001 | |
| Ln(ovarian volume) | −0.487 | 0.083 | 0.350 | <0.0001 | |
| Ln(FSH) | −0.301 | 0.079 | −0.228 | 0.0002 | |
| Ln(inhibin B) | 0.109 | 0.052 | 0.120 | 0.0360 | |
| (B) Age, volume, FSH | 0.483 | ||||
| Intercept | 2.070 | 0.257 | |||
| Age−30 | −0.040 | 0.007 | −0.342 | <0.0001 | |
| Ln(ovarian volume) | −0.529 | 0.081 | 0.379 | <0.0001 | |
| Ln(FSH) | −0.297 | 0.079 | −0.225 | 0.0002 | |
| (C) Age, volume, inhibin B | 0.453 | ||||
| Intercept | 1.139 | 0.281 | |||
| Age−30 | −0.046 | 0.007 | −0.396 | <0.0001 | |
| Ln(ovarian volume) | 0.559 | 0.084 | 0.401 | <0.0001 | |
| Ln(inhibin B) | 0.105 | 0.054 | 0.115 | 0.0520 | |
| (D) Age, FSH, inhibin B | 0.394 | ||||
| Intercept | 2.776 | 0.271 | |||
| Age−30 | −0.044 | 0.007 | −0.378 | <0.0001 | |
| Ln(FSH) | −0.406 | 0.084 | −0.307 | <0.0001 | |
| Ln(inhibin B) | 0.181 | 0.055 | 0.199 | 0.0012 | |
| (E) Age, volume | 0.441 | ||||
| Intercept | 1.499 | 0.215 | |||
| Age−30 | −0.047 | 0.007 | −0.408 | <0.0001 | |
| Ln(ovarian volume) | −0.598 | 0.082 | 0.429 | <0.0001 | |
| (F) Age, FSH | 0.356 | ||||
| Intercept | 3.582 | 0.123 | |||
| Age−30 | −0.047 | 0.008 | −0.408 | <0.0001 | |
| Ln(FSH) | −0.415 | 0.086 | −0.314 | <0.0001 | |
| (G) Age, inhibin B | 0.312 | ||||
| Intercept | 2.201 | 0.260 | |||
| Age−30 | −0.056 | 0.007 | −0.485 | <0.0001 | |
| Ln(inhibin B) | 0.190 | 0.058 | 0.208 | 0.0013 | |
| (H) Age | 0.269 | ||||
| Intercept | 3.033 | 0.050 | |||
| Age−30 | −0.060 | 0.008 | −0.529 | <0.0001 |
We centered the age variable by subtracting 30 from each woman’s age in years at ultrasound.
Summary data on selected regression modelsa
| Model . | Area under the ROC curve . | Regression equation cutpoint which yields 80% sensitivity . | Corresponding to sensitivity of 80% . | . | . | ||
|---|---|---|---|---|---|---|---|
| . | . | . | Specificity . | Positive predictive valueb . | Negative predictive valueb . | ||
| (A) Age, ovarian volume, FSH, inhibin B | 0.86 | ≤14.85 | 0.77 | 0.60 | 0.90 | ||
| (B) Age, ovarian volume, FSH | 0.85 | ≤14.30 | 0.76 | 0.58 | 0.89 | ||
| (C) Age, ovarian volume, inhibin B | 0.83 | ≤15.85 | 0.68 | 0.52 | 0.89 | ||
| (D) Age, FSH, inhibin B | 0.84 | ≤15.35 | 0.72 | 0.57 | 0.90 | ||
| (E) Age, ovarian volume | 0.82 | ≤14.95 | 0.73 | 0.55 | 0.89 | ||
| (F) Age, FSH | 0.82 | ≤15.35 | 0.66 | 0.51 | 0.89 | ||
| (G) Age, inhibin B | 0.80 | ≤15.87 | 0.63 | 0.48 | 0.89 | ||
| (H) Age | 0.77 | ≤17.10 | 0.51 | 0.42 | 0.86 | ||
| Model . | Area under the ROC curve . | Regression equation cutpoint which yields 80% sensitivity . | Corresponding to sensitivity of 80% . | . | . | ||
|---|---|---|---|---|---|---|---|
| . | . | . | Specificity . | Positive predictive valueb . | Negative predictive valueb . | ||
| (A) Age, ovarian volume, FSH, inhibin B | 0.86 | ≤14.85 | 0.77 | 0.60 | 0.90 | ||
| (B) Age, ovarian volume, FSH | 0.85 | ≤14.30 | 0.76 | 0.58 | 0.89 | ||
| (C) Age, ovarian volume, inhibin B | 0.83 | ≤15.85 | 0.68 | 0.52 | 0.89 | ||
| (D) Age, FSH, inhibin B | 0.84 | ≤15.35 | 0.72 | 0.57 | 0.90 | ||
| (E) Age, ovarian volume | 0.82 | ≤14.95 | 0.73 | 0.55 | 0.89 | ||
| (F) Age, FSH | 0.82 | ≤15.35 | 0.66 | 0.51 | 0.89 | ||
| (G) Age, inhibin B | 0.80 | ≤15.87 | 0.63 | 0.48 | 0.89 | ||
| (H) Age | 0.77 | ≤17.10 | 0.51 | 0.42 | 0.86 | ||
(i) Each model's ability to discriminate between women with low (≤10 follicles) and high (>10 follicles) antral follicle count [the area under the receiver operating characteristic (ROC) curve]; (ii) specificity and predictive values at the regression equation cutpoint yielding 80% sensitivity.
In the sample, 30.1% (53/176) of women had ≤10 antral follicles.
Summary data on selected regression modelsa
| Model . | Area under the ROC curve . | Regression equation cutpoint which yields 80% sensitivity . | Corresponding to sensitivity of 80% . | . | . | ||
|---|---|---|---|---|---|---|---|
| . | . | . | Specificity . | Positive predictive valueb . | Negative predictive valueb . | ||
| (A) Age, ovarian volume, FSH, inhibin B | 0.86 | ≤14.85 | 0.77 | 0.60 | 0.90 | ||
| (B) Age, ovarian volume, FSH | 0.85 | ≤14.30 | 0.76 | 0.58 | 0.89 | ||
| (C) Age, ovarian volume, inhibin B | 0.83 | ≤15.85 | 0.68 | 0.52 | 0.89 | ||
| (D) Age, FSH, inhibin B | 0.84 | ≤15.35 | 0.72 | 0.57 | 0.90 | ||
| (E) Age, ovarian volume | 0.82 | ≤14.95 | 0.73 | 0.55 | 0.89 | ||
| (F) Age, FSH | 0.82 | ≤15.35 | 0.66 | 0.51 | 0.89 | ||
| (G) Age, inhibin B | 0.80 | ≤15.87 | 0.63 | 0.48 | 0.89 | ||
| (H) Age | 0.77 | ≤17.10 | 0.51 | 0.42 | 0.86 | ||
| Model . | Area under the ROC curve . | Regression equation cutpoint which yields 80% sensitivity . | Corresponding to sensitivity of 80% . | . | . | ||
|---|---|---|---|---|---|---|---|
| . | . | . | Specificity . | Positive predictive valueb . | Negative predictive valueb . | ||
| (A) Age, ovarian volume, FSH, inhibin B | 0.86 | ≤14.85 | 0.77 | 0.60 | 0.90 | ||
| (B) Age, ovarian volume, FSH | 0.85 | ≤14.30 | 0.76 | 0.58 | 0.89 | ||
| (C) Age, ovarian volume, inhibin B | 0.83 | ≤15.85 | 0.68 | 0.52 | 0.89 | ||
| (D) Age, FSH, inhibin B | 0.84 | ≤15.35 | 0.72 | 0.57 | 0.90 | ||
| (E) Age, ovarian volume | 0.82 | ≤14.95 | 0.73 | 0.55 | 0.89 | ||
| (F) Age, FSH | 0.82 | ≤15.35 | 0.66 | 0.51 | 0.89 | ||
| (G) Age, inhibin B | 0.80 | ≤15.87 | 0.63 | 0.48 | 0.89 | ||
| (H) Age | 0.77 | ≤17.10 | 0.51 | 0.42 | 0.86 | ||
(i) Each model's ability to discriminate between women with low (≤10 follicles) and high (>10 follicles) antral follicle count [the area under the receiver operating characteristic (ROC) curve]; (ii) specificity and predictive values at the regression equation cutpoint yielding 80% sensitivity.
In the sample, 30.1% (53/176) of women had ≤10 antral follicles.
Summary data on the performance of five regression models in younger (<35 years) and older (≥35 years) womena
| Model . | Area under the ROC curve . | Regression equation cutpoint which yields 80% sensitivity . | Corresponding to sensitivity of 80% . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Specificity . | Positive predictive value . | Negative predictive value . | |||||
| Women <35 yearsb | ||||||||||
| (A) Age, ovarian volume, FSH, inhibin B | 0.75 | ≤22.05 | 0.39 | 0.17 | 0.92 | |||||
| (B) Age, ovarian volume, FSH | 0.77 | ≤20.05 | 0.48 | 0.21 | 0.95 | |||||
| (D) Age, FSH, inhibin B | 0.64 | ≤20.55 | 0.42 | 0.18 | 0.93 | |||||
| (E) Age, ovarian volume | 0.74 | ≤21.30 | 0.43 | 0.18 | 0.93 | |||||
| (H) Age | 0.51 | ≤22.30 | 0.25 | 0.14 | 0.88 | |||||
| Women ≥35 yearsc | ||||||||||
| (A) Age, ovarian volume, FSH, inhibin B | 0.85 | ≤11.55 | 0.77 | 0.79 | 0.78 | |||||
| (B) Age, ovarian volume, FSH | 0.82 | ≤11.55 | 0.69 | 0.74 | 0.76 | |||||
| (D) Age, FSH, inhibin B | 0.85 | ≤12.80 | 0.66 | 0.71 | 0.77 | |||||
| (E) Age, ovarian volume | 0.77 | ≤11.93 | 0.57 | 0.65 | 0.69 | |||||
| (H) Age | 0.72 | ≤12.40 | 0.42 | 0.60 | 0.68 | |||||
| Model . | Area under the ROC curve . | Regression equation cutpoint which yields 80% sensitivity . | Corresponding to sensitivity of 80% . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Specificity . | Positive predictive value . | Negative predictive value . | |||||
| Women <35 yearsb | ||||||||||
| (A) Age, ovarian volume, FSH, inhibin B | 0.75 | ≤22.05 | 0.39 | 0.17 | 0.92 | |||||
| (B) Age, ovarian volume, FSH | 0.77 | ≤20.05 | 0.48 | 0.21 | 0.95 | |||||
| (D) Age, FSH, inhibin B | 0.64 | ≤20.55 | 0.42 | 0.18 | 0.93 | |||||
| (E) Age, ovarian volume | 0.74 | ≤21.30 | 0.43 | 0.18 | 0.93 | |||||
| (H) Age | 0.51 | ≤22.30 | 0.25 | 0.14 | 0.88 | |||||
| Women ≥35 yearsc | ||||||||||
| (A) Age, ovarian volume, FSH, inhibin B | 0.85 | ≤11.55 | 0.77 | 0.79 | 0.78 | |||||
| (B) Age, ovarian volume, FSH | 0.82 | ≤11.55 | 0.69 | 0.74 | 0.76 | |||||
| (D) Age, FSH, inhibin B | 0.85 | ≤12.80 | 0.66 | 0.71 | 0.77 | |||||
| (E) Age, ovarian volume | 0.77 | ≤11.93 | 0.57 | 0.65 | 0.69 | |||||
| (H) Age | 0.72 | ≤12.40 | 0.42 | 0.60 | 0.68 | |||||
(i)Each model's ability to discriminate between women with low (≤10 follicles) and high (>10 follicles) antral follicle count [the area under the receiver operating characteristic (ROC) curve]; (ii) specificity and predictive values at the regression equation cutpoint yielding 80% sensitivity.
Among women <35 years, 13.9% (14/101) had ≤10 antral follicles.
Among women ≥35 years, 52.0% (39/75) had ≤10 antral follicles.
Summary data on the performance of five regression models in younger (<35 years) and older (≥35 years) womena
| Model . | Area under the ROC curve . | Regression equation cutpoint which yields 80% sensitivity . | Corresponding to sensitivity of 80% . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Specificity . | Positive predictive value . | Negative predictive value . | |||||
| Women <35 yearsb | ||||||||||
| (A) Age, ovarian volume, FSH, inhibin B | 0.75 | ≤22.05 | 0.39 | 0.17 | 0.92 | |||||
| (B) Age, ovarian volume, FSH | 0.77 | ≤20.05 | 0.48 | 0.21 | 0.95 | |||||
| (D) Age, FSH, inhibin B | 0.64 | ≤20.55 | 0.42 | 0.18 | 0.93 | |||||
| (E) Age, ovarian volume | 0.74 | ≤21.30 | 0.43 | 0.18 | 0.93 | |||||
| (H) Age | 0.51 | ≤22.30 | 0.25 | 0.14 | 0.88 | |||||
| Women ≥35 yearsc | ||||||||||
| (A) Age, ovarian volume, FSH, inhibin B | 0.85 | ≤11.55 | 0.77 | 0.79 | 0.78 | |||||
| (B) Age, ovarian volume, FSH | 0.82 | ≤11.55 | 0.69 | 0.74 | 0.76 | |||||
| (D) Age, FSH, inhibin B | 0.85 | ≤12.80 | 0.66 | 0.71 | 0.77 | |||||
| (E) Age, ovarian volume | 0.77 | ≤11.93 | 0.57 | 0.65 | 0.69 | |||||
| (H) Age | 0.72 | ≤12.40 | 0.42 | 0.60 | 0.68 | |||||
| Model . | Area under the ROC curve . | Regression equation cutpoint which yields 80% sensitivity . | Corresponding to sensitivity of 80% . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Specificity . | Positive predictive value . | Negative predictive value . | |||||
| Women <35 yearsb | ||||||||||
| (A) Age, ovarian volume, FSH, inhibin B | 0.75 | ≤22.05 | 0.39 | 0.17 | 0.92 | |||||
| (B) Age, ovarian volume, FSH | 0.77 | ≤20.05 | 0.48 | 0.21 | 0.95 | |||||
| (D) Age, FSH, inhibin B | 0.64 | ≤20.55 | 0.42 | 0.18 | 0.93 | |||||
| (E) Age, ovarian volume | 0.74 | ≤21.30 | 0.43 | 0.18 | 0.93 | |||||
| (H) Age | 0.51 | ≤22.30 | 0.25 | 0.14 | 0.88 | |||||
| Women ≥35 yearsc | ||||||||||
| (A) Age, ovarian volume, FSH, inhibin B | 0.85 | ≤11.55 | 0.77 | 0.79 | 0.78 | |||||
| (B) Age, ovarian volume, FSH | 0.82 | ≤11.55 | 0.69 | 0.74 | 0.76 | |||||
| (D) Age, FSH, inhibin B | 0.85 | ≤12.80 | 0.66 | 0.71 | 0.77 | |||||
| (E) Age, ovarian volume | 0.77 | ≤11.93 | 0.57 | 0.65 | 0.69 | |||||
| (H) Age | 0.72 | ≤12.40 | 0.42 | 0.60 | 0.68 | |||||
(i)Each model's ability to discriminate between women with low (≤10 follicles) and high (>10 follicles) antral follicle count [the area under the receiver operating characteristic (ROC) curve]; (ii) specificity and predictive values at the regression equation cutpoint yielding 80% sensitivity.
Among women <35 years, 13.9% (14/101) had ≤10 antral follicles.
Among women ≥35 years, 52.0% (39/75) had ≤10 antral follicles.
We acknowledge our co-investigators Drs Dorothy Warburton and Michel Ferin who collaborated in the design and implementation of the study. We thank Dr Grace Jorgensen who welcomed and facilitated this study; we thank her and her colleagues for their help in providing access to their patients. We acknowledge Maria Bautista, Jennifer Cassin, Terry Fox, the late Kris Keough, and Donna West who facilitated our work at the study hospital; Rebecca Russell and Jeannie Small-Fish who obtained the sonography scans. We thank Megan Meldrum who carried out the fieldwork of the study and Renee Davenport who assisted in data processing and checking. This research would not be possible without the help of the women who participated in it to further understanding of the causes of reproductive loss. This work was supported by a grant from the National Institutes on Aging (R01 AG 15386).
References
Baker TG (
Bancsi LF, Broekmans FJ, Mol BW, Habbema JD and te Velde ER (
Block E (
Broekmans FJ, Scheffer GJ, Bancsi LF, Dorland M, Blankenstein MA and te Velde ER (
Broekmans FJ, Faddy MJ, Scheffer G and Te Velde ER (
Bukman A and Heineman MJ (
Cramer DW, Barbieri RL, Xu H and Reichardt JK (
Danforth DR, Arbogast LK, Mroueh J, Kim MH, Kennard EA, Seifer DB and Friedman CI (
de Vet A, Laven JS, de Jong FH, Themmen AP and Fauser BC (
Erdem A, Erdem M, Biberoglu K, Hayit O, Arslan M and Gursoy R (
Faddy MJ, Gosden RG, Gougeon A, Richardson SJ and Nelson JF (
Groome NP, Illingworth PJ, O'Brien M, Pai R, Rodger FE, Mather JP and McNeilly AS (
Hansen KR, Morris JL, Thyer AC and Soules MR (
Howe G, Westhoff C, Vessey M and Yeates D (
Klein NA, Battaglia DE, Miller PB, Branigan EF, Giudice LC and Soules MR (
Klein NA, Illingworth PJ, Groome NP, McNeilly AS, Battaglia DE and Soules MR (
Klein NA, Battaglia DE, Woodruff TK, Padmanabhan V, Giudice LC, Bremner WJ and Soules MR (
Kline J, Kinney A, Reuss ML, Kelly A, Levin B, Ferin M and Warburton D (
Lee SJ, Lenton EA, Sexton L and Cooke ID (
Leidy LE, Godfrey LR and Sutherland MR (
Lenton EA, Sexton L, Lee S and Cooke ID (
Matrox Electronic Systems Ltd (2005) Matrox Inspector. Available at http://www.matrox.com/imaging (Accessed March 15, 2005).
McNeil BJ and Hanley JA (
Metcalf MG and Livesey JH (
Pavlik EJ, DePriest PD, Gallion HH, Ueland FR, Reedy MB, Kryscio RJ and van Nagell JR Jr (
Reuss ML, Kline J, Santos R, Levin B and Timor–Tritsch I (
Reyes FI, Winter JS and Faiman C (
Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW and te Velde ER (
Scheffer GJ, Broekmans FJ, Bancsi LF, Habbema JD, Looman CW and te Velde ER (
Scheffer GJ, Broekmans FJ, Looman CW, Blankenstein M, Fauser BC, teJong FH and teVelde ER (
Schipper I, de Jong FH and Fauser BC (
Schwartz D and Mayaux MJ (
Thomford PJ, Jelovsek FR and Mattison DR (
Toner JP, Grainger DA and Frazier LM (
van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH and Themmen AP (
Welt CK, McNicholl DJ, Taylor AE and Hall JE (
Author notes
1Epidemiology of Developmental Brain Disorders Department, New York State Psychiatric Institute, New York, NY 10032, 2Gertrude H. Sergievsky Center and 3Mailman School of Public Health, Columbia University, 4Research Foundation for Mental Hygiene, New York State Psychiatric Institute, New York, NY and Graduate School of Arts and Sciences, Columbia University, 5Department of Obstetrics and Gynecology, Columbia University, New York, NY 10032 and 6Southwest Women's Sonography, Albuquerque, NM 87109, USA