-
PDF
- Split View
-
Views
-
Cite
Cite
Rui Matsuura-Sawada, Takashi Murakami, Yuka Ozawa, Hiroshi Nabeshima, Jun-ichi Akahira, Yumi Sato, Yoshio Koyanagi, Mamoru Ito, Yukihiro Terada, Kunihiro Okamura, Reproduction of menstrual changes in transplanted human endometrial tissue in immunodeficient mice, Human Reproduction, Volume 20, Issue 6, June 2005, Pages 1477–1484, https://doi.org/10.1093/humrep/deh783
Close - Share Icon Share
Abstract
BACKGROUND: Cultures of human endometrial tissue are useful for analysing the mechanisms underlying the menstrual cycle. However, long-term culture of endometrial tissue is difficult in vitro. Xenotransplantation of normal human endometrial tissue into immunodeficient mice could allow prolonged survival of the transplanted tissues. METHODS: Proliferative-phase endometrial tissue samples from three women were transplanted into the subcutaneous space of ovariectomized, immunodeficient, non-obese diabetic (NOD)/severe combined immunodeficiency (SCID)/γCnull (NOG) mice. The mice were treated with 17β-estradiol (E2) for the first 14 days after transplantation, followed by E2 plus progesterone for the next 14 days. The transplants were investigated morphologically and immunohistochemically at various times after implantation. RESULTS: The transplanted tissues contained large numbers of small glands, pseudostratification of the nuclei and dense stroma after treatment with E2 alone. After treatment with E2 plus progesterone, subnuclear vacuolation, luminal secretion and decidualization of the stroma were observed. When the hormone treatment ceased, tissue destruction occurred and the transplants returned to the proliferative phase. Lymphocytes were identified immunohistochemically: the numbers of CD56-positive and CD16-negative cells increased significantly in the stroma during the late secretory phase (day 28). CONCLUSIONS: Human endometrial tissue transplanted into NOG mice showed similar histological changes to eutopic endometrial tissue during treatment with sex steroid hormones for 1 month. Moreover, lymphocytes were produced in the transplanted human endometrial tissue. This system represents a new experimental model of the human endometrium in vivo.
Introduction
Endometrial tissue undergoes periodic cycles of proliferation, differentiation and degeneration that are precisely controlled by sex steroid hormones produced in the ovaries. If fertilization does not occur during a cycle, the cells of the endometrium degenerate and slough off, and the organ prepares itself for nidation in the next cycle. These tissue dynamics have, so far, been difficult to reproduce in experimental models. Therefore, the exact mechanisms that are responsible for these processes are poorly understood.
In 1908, the earliest comprehensive description of the cyclical histological changes that occur in the human endometrium was published (Hitschmann and Adler, 1908). Subsequently, Noyes and colleagues described several histological features that are still used today as criteria for endometrial dating: gland mitoses, pseudostratification of the nuclei, subnuclear vacuolation, gland secretion, stromal oedema, stromal mitoses, pseudodecidual reactions and leukocytic infiltration (Noyes et al., 1950). Although these histological changes have been used widely in clinical diagnosis, their implications are not yet fully understood.
To understand the mechanisms of reproduction, it is essential to establish experimental models of the endometrium throughout the menstrual cycle. Many experiments have examined the mechanism of action of steroid hormones on the endometrium in laboratory animals (Jensen and Jacobson, 1962; O'Mally et al., 1970; Flickinger et al., 1977). However, in mature mice and rats, the reproductive cycle is only 4 or 5 days long and decidualization does not occur unless it is triggered by implantation or stimulated by pregnancy (Finn et al., 1992). Similarly, natural ovulation does not occur in mature rabbits unless mating has occurred. Menstruation occurs only in a very limited number of species: humans, a few Old World primates and a few bats. However, there are significant differences between, for example, rhesus monkeys and humans, in terms of the changes that occur in the endometrium at implantation (Heuser et al., 1945) and the distribution of the aortic branches supplying the uterus (Nelson, 1964). The reproductive cycle, biochemical responses and mechanisms of menstruation clearly differ between species. Therefore, the results of studies in laboratory animals have been difficult to apply directly to humans.
There have been several studies of the human endometrium using in vitro endometrial stroma and/or glandular cell cultures over the past two decades (Centola et al., 1984; Irwin et al., 1989; Sugawara et al., 1997). Although these studies have produced many interesting findings, they may not accurately reflect the events that occur in vivo. Tissue culture is a useful method for biochemical and histological analyses. However, it is difficult to culture human endometrial tissue over long periods of time—bearing in mind the 1-month duration of each menstrual cycle—in order to observe the relationship between the glandular and stromal cells.
On the other hand, there have been many reports of successful transplantation of human tissues into immunodeficient mice. Nude mice, which lack a thymus and cannot generate mature T lymphocytes, were first used as recipients of xenotransplants of human adenocarcinoma of the sigmoid colon (Rygaard and Povlsen, 1969), and severe combined immunodeficiency (SCID) mice, which are deficient in both T and B lymphocytes, were used as a model of human stem-cell engraftment (McCune et al., 1988). Subsequently, non-obese diabetic (NOD)-SCID mice were developed, which have a higher acceptance rate not only for neoplastic tissue but also for tissue from normal human organs. Weissman and colleagues successfully transplanted normal human ovarian cortex tissue into NOD-SCID mice and observed primordial follicle growth (Weissman et al., 1999). In addition, Sato and colleagues detected by immunohistochemistry the expression of steroidogenic enzymes in NOD-SCID mice after transplantation of human ovarian grafts (Sato et al., 2003). However, although these mice lack T and B lymphocytes, they do have some natural killer (NK) cell activity, which might interfere with engraftment efficiency.
Recently, NOD/SCID/γCnull (NOG) immunodeficient mice have been developed; these are double homozygotes for the SCID mutation and the interleukin-2 receptor γ-chain (IL-2Rγ) allelic mutation (γcnull). NOG mice lack both T and B lymphocytes, and are defective in NK-cell activity. This severe immunodeficiency results in high engraftment efficiency for human haematopoietic stem cells and full lineage differentiation in NOG mice (Ito et al., 2002).
The aims of the present study were to culture human endometrial tissue in NOG mice, and to reproduce menstrual changes by examining their morphological and immunohistochemical features.
Materials and methods
Ethical approval
All procedures for collecting human specimens and all animal experiments were approved by the Ethics Committee of Tohoku University Graduate School of Medicine, Japan.
Animals
Mature female NOG mice aged 7–8 weeks and weighing 20–25 g were obtained from the Central Institute for Experimental Animals (Kawasaki, Japan). The animals were housed in micro-isolator cages in a barrier facility under well controlled, pathogen-free conditions. The monitored ambient temperature was 22 °C and the animals were maintained under a 12-h light/dark cycle. All housing materials were autoclaved before use. The mice were fed laboratory chow and water ad libitum.
Human endometrial tissue
Human endometrial tissues at the proliferative phase of the menstrual cycle were obtained from three premenopausal women (aged 35–49 years) who were undergoing hysterectomy as a result of the benign gynaecological disease, myoma uteri, at Tohoku University Hospital, Japan. Informed consent was obtained from each patient. No lesions of endometriosis were found in the abdomen of the patients during surgery. Each of the patients had a regular menstrual cycle, which had reached the early proliferative phase at the time of surgery; this was confirmed by measuring serum concentrations of 17β-estradiol (E2) and progesterone. A sample of the endometrial tissue was fixed in 10% neutral buffered formalin, embedded in paraffin, and stained with haematoxylin and eosin in order to determine the menstrual phase according to the method of Noyes et al. (1950).
Fresh endometrial tissue was collected in cold sterile Dulbecco's phosphate-buffered saline, cut into fragments (diameter 2 mm) with a safety razor blade and washed twice to remove cellular debris.
Transplantation of endometrial fragments into mice
Eighteen NOG mice were placed under NEMBUTAL (Dainippon Pharmaceutical Co., Ltd, Osaka, Japan) anaesthesia by intraperitoneal injection. A small dorsolateral laparotomy was created in the abdomen of each mouse, and a bilateral ovariectomy was performed to prevent the sex hormones of the animals having an effect on the transplanted tissue. After ovariectomy, two fragments of the human endometrial tissue were transplanted into the subcutaneous space of each mouse. The ovariectomy and transplantation procedure was carried out within 3 h of hysterectomy. The treatment with sex hormones was initiated at the time of tissue transplantation. E2 (FEMIEST; Yakult Honsha Co., Ltd, Tokyo, Japan) was administered to the mice using a transdermal patch, and progesterone (Progehormone; Mochida Pharmaceutical Co., Ltd, Tokyo, Japan) was administered by subcutaneous injection. The FEMIEST patches were cut into 0.64- or 1-cm2 sections and attached to the backs of the mice in areas from which the fur had been removed. Mice received E2 alone for the first 14 days (0.64 cm2 containing 0.2 mg of E2 for the first 7 days, and 1 cm2 containing 0.3 mg of E2 for the following 7 days), and E2 (0.64 cm2) plus progesterone (0.5 mg/kg) for the next 14 days. The patches were changed every 3 days. Hormone administration was stopped after the 28-day treatment period. All procedures were performed under aseptic conditions in a clean-bench environment. The animals were maintained for a maximum of 35 days without antibiotics.
Histological assessment
Mice were sacrificed by removing blood from the heart under ether anaesthesia at 14, 16, 21, 28, 31 or 35 days after transplantation. The implanted endometrial fragments were extracted and fixed in 10% neutral buffered formalin for ∼24 h, then embedded in paraffin. Sections (thickness 3 μm) were stained with haematoxylin and eosin for histological identification. Serum samples were measured using an enzyme-linked immunosorbent assay (Cayman Chemical Co., Ann Arbor, MI, USA).
Immunohistochemical analyses
Single immunohistochemical labelling. Immunohistochemical analyses were performed in order to demonstrate proliferative activity and to determine the type of lymphocytes that appeared in the human endometrium. The primary human antibodies used in these analyses are summarized in Table I. A HISTFINE kit (Nichirei, Tokyo, Japan) and an EnVision kit (DakoCytomation, Inc., Carpinteria, CA, USA) were used to identify the human lymphocytes. Sections (1.5 μm) were deparaffinized and treated with methanol/3% hydrogen peroxide to block endogenous peroxidase. In order to retrieve masked antigens, the slides were immersed in citrate buffer (pH 6.0) and heated in an autoclave for 5 min at 121 °C. They were then incubated with primary antibody overnight, followed by biotinylated secondary antibody for 30 min and peroxidase-labelled streptavidin for 30 min. The antigen–antibody complex was visualized with 3,3′-diaminobenzidine (DAB) solution and counterstained with haematoxylin. Positive controls were samples of endometrial cancer (Ki-67), small-cell carcinoma (CD56), thymus (CD16) and normal human lymph nodes (CD3 and CD79α). The pairs of mirror-image sections were obtained simultaneously and stained for CD56 and CD16.
Double immunohistochemical labelling. The sections of day 28 were also labelled using a sequential double immunohistochemical staining for CD56 and CD3. Sections were incubated with CD56 overnight at first and the reaction was developed with DAB. After that, sections were incubated with CD3 overnight, and the reaction was developed with 4-chloro-1-naphthol.
Results
Transplantation
All of the NOG mice that received transplants of normal human endometrial tissue into the subcutaneous space survived and were sacrificed between 14 and 35 days after surgery. The success rate of xenotransplantation was 100% and the recovery rate of fragments was 94%. The two fragments transplanted into each mouse showed similar histological changes, which were not dependent upon the patient.
Histological assessment
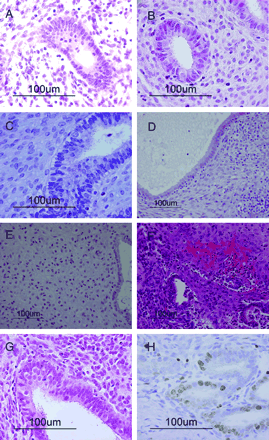
Before transplantation, the endometrial tissue contained numerous small narrow glands with columnar glandular cells. Evidence of pseudostratification of the nuclei was also observed. The stroma was dense, and mitotic figures were identified in both the glandular and stromal cells. These findings indicate that the endometrial tissue was in the early proliferative phase (Figure 1A).
After 14 days, the tissues contained numerous small narrow glands with columnar glandular cells and pseudostratification of the nuclei. The stroma was dense, and many mitotic figures were observed in both the glandular and stromal cells (Figure 1B). These findings suggest that the cells were actively proliferating. The mean serum concentration of E2 was 293.1 pg/ml at this stage.
By contrast, progressive development of secretory-phase characteristics was observed after treatment commenced with progesterone plus E2. Subnuclear vacuolation of the glandular epithelium, the first detectable feature of the secretory phase, began to appear after 2 days of E2 plus progesterone treatment (Figure 1C). The mean serum concentrations of E2 and progesterone were 115 pg/ml and 12.0 ng/ml, respectively. By 21 days after transplantation, the glands were noticeably dilated and filled with fluid. In addition, the glandular cells appeared cuboidal and the pseudostratification of the nuclei had disappeared. Many lymphocytes were present throughout the stroma and tended to aggregate around the glands (Figure 1D). The mean serum concentrations of E2 and progesterone were 158 pg/ml and 19.2 ng/ml, respectively. Progressive decidual change occurred in the stroma and marked decidualization was observed 28 days after transplantation. The typical late-secretory structure was present by this stage and numerous lymphocytes were identified throughout the stroma (Figure 1E). The mean serum concentrations of E2 and progesterone were 54.5 pg/ml and 16.5 ng/ml, respectively.
After 28 days hormone treatment was stopped, the decidual change ceased and tissue destruction accompanied by bleeding was observed in the stroma (Figure 1F). These findings suggest the occurrence of menstruation. By day 31, the mean serum concentrations of E2 and progesterone were 0.9 pg/ml and 0.5 ng/ml, respectively. However, by day 35 the tissues contained many small narrow glands with columnar cells and pseudostratification of the nuclei was detected (Figure 1G). The stromal cells were dense, and mitotic figures were detected in both the glandular and stromal cells, suggesting the return of the proliferative phase.
Immunohistochemical analyses
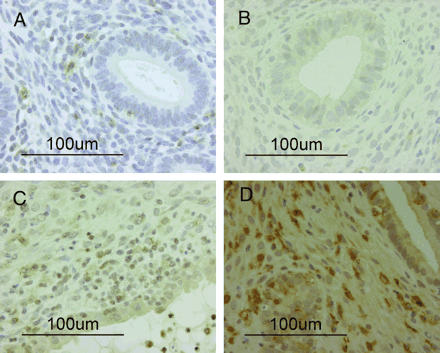
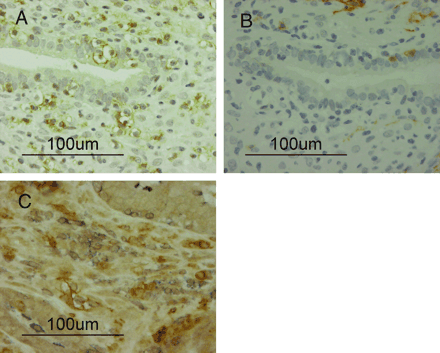
A number of nuclei in the glandular and stromal cells were stained with Ki-67 on day 35 after transplantation (Figure 1H). Human CD56-positive cells were detected in small numbers before transplantation (Figure 2A); however, these had disappeared from the stroma by day 14 (Figure 2B). A few human CD56-positive cells were again observed in the stroma at day 21 (Figure 2C) and their numbers had significantly increased by day 28 (Figure 2D). Moderate numbers of human CD3-positive cells were detected before transplantation and at day 14; however, they had increased by days 21 and 28. Small numbers of human CD16-positive cells were detected in all specimens throughout the menstrual cycle; in contrast, human CD79a-positive cells were barely detected at any stage. Moreover, CD56-positive cells (Figure 3A) did not stain for CD16 (Figure 3B), and CD56-positive cells hardly stained for CD3 (Figure 3C) by single immunohistochemical staining of paired mirror-image sections for CD56 and CD16 and double staining for CD56 and CD3.
Discussion
We have demonstrated that human endometrial tissue transplanted into the subcutaneous space of NOG mice responded to sex-hormone treatment by showing the normal cyclical changes observed in the human endometrium. The recovery rate of the fragments was 94%, and the failures were probably caused by the transplants being too small to detect at day 35 (one contained only mouse tissue and the other only stromal tissue of human endometrium).
Several previous workers have transplanted human endometrial tissue into immunodeficient mice (Zamah et al., 1984; Bergqvist et al., 1985; Zaino et al., 1985; Aoki et al., 1994; Awwad et al., 1999; Tabibzadeh et al., 1999; Nisolle et al., 2000a; b; Grummer et al., 2001; Beliard et al., 2002; Hull et al., 2003). These experiments were highly variable with respect to the strain of mouse used, the phase of the menstrual cycle of the transplanted tissue, the site of transplantation, whether or not ovariectomy was performed, whether or not the endometrium was pretreated and the method by which sex hormones were administered. However, despite these differences, most previous studies concluded that this system was a suitable model of endometriosis.
Zaino and colleagues transplanted human endometrial tissue into the subcutaneous space of ovariectomized athymic mice in four treatment groups (E2, E2 and progesterone, progesterone, and no exogenous hormone) and suggested that their model was useful for examining the histological response of normal endometrial tissue to sex hormones (Zaino et al., 1985). The novelty of the approach described in the present report involves the simultaneous administration of sex steroids similar to those involved in the human menstrual cycle. Moreover, our model utilizes NOG mice that lack both T and B lymphocytes and NK-cell activity.
We observed many small narrow glands with columnar cells and pseudostratification of the nuclei in the grafted tissue 14 days after transplantation. Treatment with E2 plus progesterone resulted in the following sequence of secretory changes in the endometrial tissue: subnuclear vacuolation and glandular secretion, followed by notable dilation of the glands, the presence of cuboidal glandular cells and the gradual decidualization of the stromal cells. This corresponds to the normal secretory phase of the endometrium. Furthermore, lymphocytes were observed during E2 plus progesterone treatment in the mid-to-late secretory phase. After the sex-hormone treatment had ceased, tissue destruction and bleeding were observed in the stroma. We subsequently observed the reconstruction of the endometrium, the presence of small glands with columnar cells, pseudostratification of the nuclei, and mitoses in both the glandular and stromal cells. The proliferative activity was confirmed by immunohistochemistry. Ferenczy (1976) demonstrated that reconstruction of the eutopic endometrium during the early stage of the menstrual cycle is independent of ovarian hormone. Our findings suggest that the transplanted human endometrial tissue in our model returned to the proliferative phase, despite the low concentration of E2.
The endometrial tissue in our system contained numerous lymphocytes at 21 and 28 days after transplantation. The number of large granular lymphocytes in human endometrial tissue has been reported to increase during the mid to late secretory phase, and CD56-positive, CD16-negative uterine NK cells have been identified (King et al., 1989; Bulmer et al., 1991; Klentzeris et al., 1992) We detected small numbers of CD56-positive and CD16-negative cells on day 21, and their numbers had increased by day 28. These cells showed very little CD3 staining. It was also reported that CD3-positive cells increased from 4–7 days after the LH surge, and remained unchanged in number after that (Klentzeris et al., 1992). In this model, moderate numbers of CD3-positive lymphocytes were detected at day 14; they increased on day 21 and similar numbers of these cells were detected on day 28. From these points of view, our model successfully maintained the human endometrial structure and reproduced normal menstrual changes. It is therefore suitable for studying the human endometrium both from a histological perspective and from the appearance of lymphocytes, such as CD56-positive NK cells and CD3-positive cells.
Dallenbach-Hellweg (1971) suggested that endometrial granulated lymphocytes develop from undifferentiated endometrial stromal cells. In this study, the human CD56-positive cells that were present in the stroma before transplantation completely disappeared by day 14, reappeared at day 21 and had significantly increased in number by day 28. As the mice used in this study lacked B and T lymphocytes and NK cells, these cells could not have been derived from the peripheral blood of the mice. Therefore, there are two possibilities of the origin of CD56-positive NK cells: one is that they differentiated from the transplanted endometrial stromal cells, the other is precursor cells were located within the transplanted endometrial tissue and they arose and differentiated gradually. It could be possible to investigate the origin of the uterine NK cells by using this model; however, further studies will be necessary to clarify this issue.
We noted some differences between the transplanted tissue in our model and the normal human endometrium. Spiral arteries were not observed in the transplants after either 21 or 28 days; this was probably due to the fragmentary nature of the endometrial tissues. Normally, the uterine arteries penetrate the uterine wall and advance into the middle layer of the myometrium, where they ramify into arcuate arteries. These divide into radial arteries, which, in turn, divide into basal arteries near the myoendometrial junction. The basal arteries ascend through the stratum functionale to the endometrial surface, where they become spiral arteries. However, the transplants comprised endometrial tissue only, and lacked arcuate and radial arteries. This resulted in a different pattern of vascularization to that of normal endometrial tissues, with an absence of spiral arteries. Menstruation has long been recognized as an ischaemic necrosis of the endometrium caused by contraction of the spiral arteries due to a decrease in the levels of the sex steroid hormones (Markee, 1940; Bartelmez, 1957). However, Hoopwood and Levison (1976) reported the presence of apoptotic bodies in the human endometrium, and recent reports have also pointed to the involvement of apoptosis in the endometrium during the menstrual cycle (Tabibzadeh, 1995; Kokawa et al., 1996). Igarashi et al., (2001) confirmed the increase of CD56-positive cells in the late secretory phase and suggested that CD56-positive NK cells induce the apoptosis of endometrial glandular cells associated with the onset of menstruation. Marbaix et al., (1996) also demonstrated in vitro that matrix metalloproteinases were involved in the initiation of menstruation. In the present study, bleeding and tissue destruction were observed in the stroma 31 days after transplantation. These findings suggest that our model is suitable for investigating the mechanism of menstruation in the absence of spiral arteries.
We were careful to use only natural sex steroid hormones in our model, and the appropriate periods of administration and doses were determined in a preliminary study using one piece of endometrial tissue. Interestingly, 7 days of pretreatment with E2 followed by 14 days of E2 plus progesterone treatment did not result in decidualization of the stromal cells, despite the administration of high E2 and progesterone concentrations. Furthermore, after treatment with E2 for 28 days, the transplants largely comprised dilated glands that resembled endometrial hyperplasia. Therefore, 14 days was selected as the appropriate period of E2 administration in order to reproduce the proliferative and late secretory phases of the normal endometrium. These findings indicate that our animal model might also be useful for studying endometrial dysfunction or hyperstimulation due to under- or overexposure to estrogen.
In conclusion, we have developed a mouse model of the human menstrual hormone cycle and the recurring changes that take place in the endometrium. This novel approach may be useful for identifying the mechanism of action of steroid hormones, the origin of uterine NK cells and the mechanism of initiation of menstruation, all of which have yet to be clarified.
Histological sections stained with haematoxylin and eosin (A–G) and the immunohistochemical stain Ki-67 (H). (A) Pretransplantation endometrial tissue: the proliferative phase. The glands are small straight and narrow, with columnar glandular cells and prominent pseudostratification of the nuclei. The stromal cells are dense (magnification ×400). (B) Endometrial tissue 14 days after transplantation: E2 has been administered for 14 days. The glands are small and narrow, with tall columnar cells. Evidence of pseudostratification of the nuclei is present. The stromal cells are dense (magnification ×400). (C) Endometrial tissue 16 days after transplantation: E2 has been administered for 14 days, followed by E2 plus progesterone for 2 days. The glands still show pseudostratified structures but they have begun to enlarge, and subnuclear vacuolation of the glandular cells is visible. The stromal cells remain dense (magnification ×400). (D) Endometrial tissue 21 days after transplantation: E2 has been administered for 14 days, followed by E2 plus progesterone for 7 days. The glands are significantly dilated, the glandular cells are cuboidal and the pseudostratification of the nuclei has disappeared. Stromal decidualization is beginning. Many lymphocytes are present throughout the stroma and are aggregating around the glands (magnification ×200). (E) Endometrial tissue 28 days after transplantation: E2 has been administered for 14 days, followed by E2 plus progesterone for 14 days. The glandular cells are cuboidal. Evidence of stromal decidualization is clearly seen and lymphocytes are present in the stroma (magnification ×200). (F) Endometrial tissue 31 days after transplantation: E2 had been administered for 14 days, followed by E2 plus progesterone for 14 days, and then no hormones for the remaining 3 days. The glands and endometrial stroma have collapsed during the evolution of the transplant. There is prominent bleeding in the stroma (magnification ×200). (G) Endometrial tissue 35 days after transplantation: E2 had been administered for 14 days, followed by E2 plus progesterone for 14 days, and then no hormones for the remaining 7 days. The glands are small and narrow with tall columnar cells. Evidence of pseudostratification of the nuclei is present. The stromal cells are dense (magnification ×400). (H) Endometrial tissue 35 days after transplantation: a number of nuclei have been immunohistochemically stained with Ki-67, showing proliferation of glandular and stromal cells (magnification ×400).
Immunohistochemical staining of human CD56. (A) Pretransplantation endometrial tissue: human CD56-positive cells are present in small numbers (magnification ×400). (B) Day 14 after transplantation: human CD56-positive cells have completely disappeared from the stroma (magnification ×400). (C) Day 21 after transplantation: human CD56-positive cells are present in small numbers (magnification×400). (D) Day 28 after transplantation: human CD56-positive cells have significantly increased in number in the stroma (magnification ×400).
Paired mirror-image sections for CD56 (A) and CD16 (B), and double immunohistochemical staining section for CD56 and CD3 (C) at day 28: There are many CD56-positive cells in the stroma; however, they are not stained with CD16. There are many CD56-positive cells and also many CD3-positive cells in the stroma (brown deposit shows CD56-positive cells and the blue CD3-positive cells); there was minimal overlap in labelling for CD56 and CD3 (magnification ×400).
Summary of the primary antibodies used in this study
| Antibody (clone number) . | Source . | Optimal dilution . | Antigen retrieval . |
|---|---|---|---|
| Ki-67 (monoclonal: MIB-1) | Dako (Glostrup, Denmark) | 1:50 | Autoclavea |
| CD56 (monoclonal: 123C3) | Monosan (Am uden, The Netherlands) | 1:80 | Autoclavea |
| CD16 (monoclonal: 2H7) | Novo Castra (Newcastle, UK) | 1:160 | Autoclavea |
| CD3 (polyclonal) | Dako (Glostrup, Denmark) | 1:500 | Autoclavea |
| CD79α (monoclonal: JCB117) | Dako (Glostrup, Denmark) | 1:40 | Autoclavea |
| Antibody (clone number) . | Source . | Optimal dilution . | Antigen retrieval . |
|---|---|---|---|
| Ki-67 (monoclonal: MIB-1) | Dako (Glostrup, Denmark) | 1:50 | Autoclavea |
| CD56 (monoclonal: 123C3) | Monosan (Am uden, The Netherlands) | 1:80 | Autoclavea |
| CD16 (monoclonal: 2H7) | Novo Castra (Newcastle, UK) | 1:160 | Autoclavea |
| CD3 (polyclonal) | Dako (Glostrup, Denmark) | 1:500 | Autoclavea |
| CD79α (monoclonal: JCB117) | Dako (Glostrup, Denmark) | 1:40 | Autoclavea |
Autoclave: heat in an autoclave for 5 min in citric acid buffer.
Summary of the primary antibodies used in this study
| Antibody (clone number) . | Source . | Optimal dilution . | Antigen retrieval . |
|---|---|---|---|
| Ki-67 (monoclonal: MIB-1) | Dako (Glostrup, Denmark) | 1:50 | Autoclavea |
| CD56 (monoclonal: 123C3) | Monosan (Am uden, The Netherlands) | 1:80 | Autoclavea |
| CD16 (monoclonal: 2H7) | Novo Castra (Newcastle, UK) | 1:160 | Autoclavea |
| CD3 (polyclonal) | Dako (Glostrup, Denmark) | 1:500 | Autoclavea |
| CD79α (monoclonal: JCB117) | Dako (Glostrup, Denmark) | 1:40 | Autoclavea |
| Antibody (clone number) . | Source . | Optimal dilution . | Antigen retrieval . |
|---|---|---|---|
| Ki-67 (monoclonal: MIB-1) | Dako (Glostrup, Denmark) | 1:50 | Autoclavea |
| CD56 (monoclonal: 123C3) | Monosan (Am uden, The Netherlands) | 1:80 | Autoclavea |
| CD16 (monoclonal: 2H7) | Novo Castra (Newcastle, UK) | 1:160 | Autoclavea |
| CD3 (polyclonal) | Dako (Glostrup, Denmark) | 1:500 | Autoclavea |
| CD79α (monoclonal: JCB117) | Dako (Glostrup, Denmark) | 1:40 | Autoclavea |
Autoclave: heat in an autoclave for 5 min in citric acid buffer.
The authors wish to thank Drs A. Endo and S. Uehara for their kind support, and Mr S. Okamoto and Ms K. Abe for technical assistance. They also wish to thank the patients who made such a valuable contribution to this study.
References
Aoki D, Katsuki Y, Shimizu A, Kakinuma C and Nozawa S (
Awwad JT, Sayegh RA, Tao XJ, Hassen T, Awwad ST and Isaacsos K (
Bartelmez GW (
Beliard A, Noel A, Goffin F, Frankenne F and Foidart JM (
Bergqvist A, Jeppsson S, Kullander S and Ljungberg O (
Bulmer JN, Morrison L, Longfellow M, Ritson A and Pace D (
Centola GM, Cisar M and Knab DR (
Dallenbach-Hellweg G (
Ferenczy A (
Finn CA, Pope MD and Milligan SR (
Flickinger GL, Elsner C, Illingworth DV, Muechler EK and Mikhail G (
Grummer R, Schwarzer F, Bainczyk K, Hess-Stumpp H, Regidor PA, Schindler AE and Winterhager E (
Heuser CH, Rock J and Hertig AT (
Hitschmann F and Adler L (
Hoopwood D and Levison DA (
Hull ML, Charnock-Jones DS, Chan CLK, Bruner-Tran KL, Osteen KG, Tom BDM, Fan TD and Smith SK (
Igarashi T, Konno R, Okamoto S, Moriya T, Sato S and Yajima A (
Irwin JC, Kirk D, King RJ, Quigley MM and Gwatkin RB (
Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugawara K, Tsuji K et al. (
Jensen EV and Jacobson HI (
King A, Wellings V, Gardner L and Loke YW (
Klentzeris LD, Bulmer JN, Warren A, Morrison L, Li TC and Cooke ID (
Kokawa K, Shikone T and Nakano R (
Marbaix E, Kokorine I, Moulin P, Donnez J, Eeckhout Y and Courtoy PJ (
Markee JE (
McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M and Weissman IL (
Nelson S (
Nisolle M, Casanas-Roux F and Donnez J (
Nisolle M, Casanas-Roux F, Marbaix E, Jadoul P and Donnez J (
O'Mally BW, Sherman MR and Toft DO (
Rygaard J and Povlsen CO (
Sato Y, Terada Y, Utsunomiya H, Koyanagi Y, Ito M, Miyoshi I, Suzuki T, Sasano H, Murakami T, Yaegashi N et al. (
Sugawara J, Fukaya T, Murakami T, Yoshida H and Yajima A (
Tabibzadeh S (
Tabibzadeh S, Miller S, Dodson WC and Satyaswaroop PG (
Weissman A, Gotlieb L, Colgan T, Jurisicova A, Greenblatt EM and Casper RF (
Zaino RJ, Satyaswaroop PG and Mortel R (
Author notes
1Tohoku University Graduate School of Medicine – Obstetrics and Gynecology, Seiryo-machi1-1, Aoba-ku, Sendai Japan, 2Virus Research, Kyoto University – Laboratory of Viral Pathogenesis, Kyoto and 3Central Institute for Experimental Animals, Kawasaki, Japan
- hormones
- cd56 antigens
- bodily secretions
- cell nucleus
- immunologic deficiency syndromes
- lymphocytes
- menstrual cycle
- menstruation
- neural cell adhesion molecules
- reproductive physiological process
- severe combined immunodeficiency
- gonadal steroid hormones
- endometrium
- mice
- progesterone
- transplantation
- decidualization
- antigens, cd16
- vacuolation